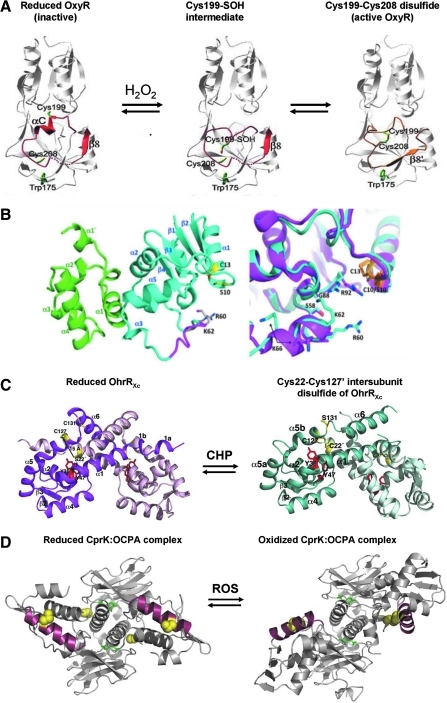
FIG. 9.
Structures of reduced and oxidized forms of the thiol-based redox sensors OxyR, Spx, OhrR (2-Cys), and CprK. (A) Structures are shown for OxyR in its reduced, Cys199 sulfenic acid intermediate and Cys199-Cys208 intramolecular disulfide conformations. In reduced OxyR, C199 and C208 are separated by 17 Å. The interaction of C199 with the side chains of T100 and R266 stabilizes the C199 thiolate anion. The residues 195 to 223, including αC and β8, are shown in red in reduced and gold in oxidized OxyR and are involved in the structural transition on oxidation. C199 is initially oxidized to the sulfenic acid intermediate that rapidly reacts further to form an intramolecular disulfide with Cys208. During OxyR oxidation, the αC helix melts, and the αC/β8 loop shifts positions. This figure is adapted from (47). (B) Structures are shown for reduced SpxC10S in complex with the αCTD. Left: Spx and αCTD are shown as teal and green ribbons, respectively. Helix α4 is colored magenta in oxidized Spx. Residues S10 and C13 are shown as sticks with carbon and sulfur atoms colored yellow and the γ-oxygen of S10, red. Right: Close-up of the region surrounding helix α4 and residues C10/S10 and C13 after the superposition of the oxidized and reduced αCTD-Spx complex structures. Reduced Spx is shown as a magenta ribbon, and oxidized Spx, as a teal ribbon. The C10-C13 disulfide bond is shown in orange sticks, and S10 and C13 from the reduced structure are shown as yellow sticks. This figure was reproduced from (62). (C) The structures of the 2-Cys OhrR of Xanthomonas campestris OhrRXc in its reduced form (left) and oxidized Cys22-Cys127′ intersubunit disulfide-linked state (right). In reduced OhrR, one subunit is colored magenta, and the other, light pink. In the reduced form, Cys22 and Cys127′ are separated by 15.5 Å. The hydrogen bonds provided by the Y36′ and Y46′ side chains (red) are involved in stabilization of the Cys22 thiolate (yellow). In the oxidized intersubunit disulfide version of the OhrR, one subunit is colored teal, and the other, light blue. On oxidation of OhrR, Cys22 is oxidized to the Cys22-SOH intermediate, disrupting the Y36′-C22-Y47′ hydrogen-bonding network and distorting α5. This allows the movement of Cys127′ by 135-degrees rotation and 8-Å translation, formation of the Cys22-Cys127′ intersubunit disulfide bond, and α6-α6′ helix-swapped reconfiguration, resulting in 28-degree rigid body rotations of the HTH motifs and dissociation from the DNA. This figure is reproduced from (64). (D) The Desulfitobacterium hafniense CprK structure is shown in ligand (OCPA) bound, reduced complex (left) and in the oxidized complex (right) containing intersubunit disulfides between C11 and C200′ (in the DNA-binding HTH motif). The C11 and C200 cysteines are indicated by yellow spheres, the bound OCPA ligand is shown in stick form (green), and the DNA-recognition helix is in red. Note that in the reduced form (left), the two DNA-binding helices are approximately parallel and appropriately spaced for interaction with their cognate DNA operator. Oxidation distorts the protein, disrupting the DNA interaction surface and preventing transcription activation. For further details of the structural changes induced in CprK by ligand binding and protein oxidation, please see (37, 54). This figure is kindly provided by Dr. David Leys, Manchester, UK.