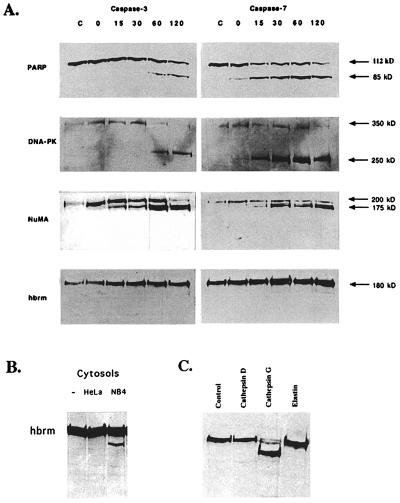
Figure 2.
Incubation of HeLa cell nuclei with purified, active caspases and cathepsins. (A) HeLa cell nuclei were prepared and resuspended in 100 μl of homogenization buffer as described in Materials and Methods. Then 100 ng of purified, active caspase-3 or caspase-7 was added to each 100-μl sample, and the samples were incubated at 30°C for the times indicated above the lanes. Control samples were incubated 2 h at 30°C without added caspase. Immediately after incubation, nuclei were repelleted and then lysed in a small volume of Nonidet P-40 lysis buffer for 30 min at 4°C. The Nonidet P-40 lysates were cleared of DNA by spinning 10 min at 10,000 × g and then used for Western blotting. (B) HeLa nuclei were incubated at 30°C for 2 h in buffer, HeLa cytosol, or NB4 cytosol, as indicated above the lanes. (C) Nuclei were incubated with buffer, purified cathepsin G, cathepsin D, or elastin as indicated.