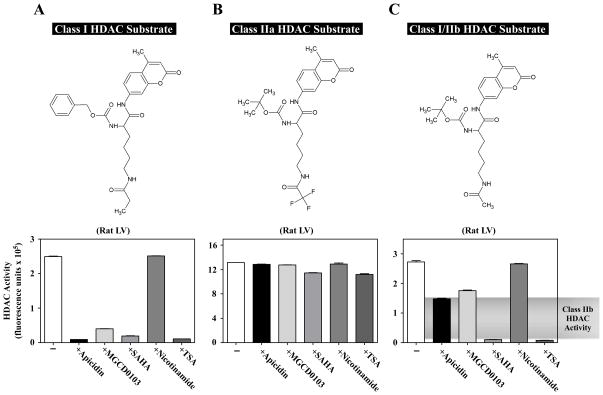
Fig. 2.
Quantification of class I, IIa and IIb HDAC catalytic activity in cardiac tissue homogenate. Rat LV extract was incubated with different HDAC substrates in the absence or presence of individual HDAC inhibitors: apicidin (3 μM), MGCD0103 (10 μM), SAHA (10 μM), nicotinamide (1 mM), or TSA (1 μM). Apicidin and MGCD0103 are class I-specific inhibitors. SAHA and TSA inhibit class I and IIb enzymes, and nicotinamide inhibits the sirtuins (class III). (A) Class I-selective substrate and corresponding HDAC activity data. In this case, apicidin and MGCD0103 behaved similar to the classical pan-inhibitors, SAHA and TSA. (B) Class IIa substrate and corresponding HDAC activity data. None of the inhibitors had a significant impact on the measured activity, confirming that this activity was not from class I, IIb, or III HDAC enzymes. (C) Class I/IIb substrate and corresponding activity data. Note that class I inhibitors lowered the activity by about 50%, revealing class IIb activity. All of the activity with this substrate was inhibited by SAHA and TSA, while nicotinamide had little effect.