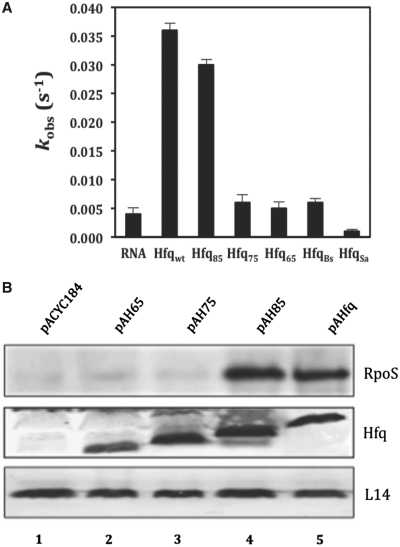
Figure 1.
The region between amino acids 75 and 85 is instrumental for RNA binding and in vivo function of HfqEc. (A) RNA annealing activities of HfqBc, HfqSa, full-length HfqEc and C-terminally truncated variants thereof. Five nanomolar single-stranded, complementary 21-nt-long oligoribonucleotides with fluorophores at their 5′-end were annealed at 37°C in the absence or presence of 100 nM protein. Relative fluorescence resonance energy transfer was calculated as the ratio of acceptor to donor fluorescence (FCy5/FCy3) as described in ‘Materials and Methods’ section. The time-resolved curves were least-square fitted with the second-order reaction equation for equimolar initial reactant concentrations: y = A[1 − (kobs t + 1)−1]; y = fraction annealed, kobs = observed annealing reaction constant, A = maximum reaction amplitude. The reaction rate kobs was calculated from the average of three independent experiments. (B) RpoS protein synthesis in the E. coli hfq- strain AM111 harboring plasmids pACYC184 (control, lane 1), pAH65 (encoding HfqEc65; lane 2), pAH75 (encoding HfqEc75; lane 3), pAH85 (encoding HfqEc85; lane 4) and pAHfq (encoding full-length HfqEc; lane 5), respectively. The western-blot analysis was carried out with anti-RpoS, anti-Hfq antibodies and with antibodies against ribosomal protein L14 (loading control) using equal amounts of total cellular protein as described in ‘Materials and Methods section’. Only the relevant sections of the western-blot showing the RpoS-, Hfq- and L14-specific signals are shown.