Abstract
The 279-bp major breakpoint region (mbr) within the 3′-untranslated region (3′-UTR) of the BCL2 gene is a binding site of special AT-rich sequence binding protein 1 (SATB1) that is well known to participate in the long-range regulation of gene transcription. Our previous studies have revealed that the mbr could regulate BCL2 transcription over a 200-kb distance and this regulatory function was closely related to SATB1. This study is to explore the underlying mechanism and its relevance to cellular apoptosis. With chromosome conformation capture (3C) and chromatin immunoprecipitation (ChIP) assays we demonstrated that the mbr could physically interact with BCL2 promoter through SATB1-mediated chromatin looping, which was required for epigenetic modifications of the promoter, CREB accessibility and high expression of the BCL2 gene. During early apoptosis, SATB1 was a key regulator of BCL2 expression. Inhibition of SATB1 cleavage by treatment of cells with a caspase-6 inhibitor or overexpression of mutant SATB1 that was resistant to caspase-6, inhibited disassembly of the SATB1-mediated chromatin loop and restored the BCL2 mRNA level in Jurkat cells. These data revealed a novel mechanism of BCL2 regulation and mechanistically link SATB1-mediated long-range interaction with the regulation of a gene controlling apoptosis pathway for the first time.
INTRODUCTION
BCL2 proto-oncogene was first cloned from the t(14;18) translocation breakpoint in human follicular B-cell lymphoma. It is ∼250 kb in length and composed of three exons and two promoters (1). The BCL2 protein is a key regulator of apoptosis and is additionally involved in DNA repair, cell cycle and differentiation control (1–5). Given its fundamental importance for the cellular fate, BCL2 expression is finely tuned by a variety of environmental and endogenous stimuli and regulated at both transcriptional and post-transcriptional levels. Our previous report has demonstrated that the major breakpoint region (mbr) within the 3′-UTR of the BCL2 gene that is 200-kb downstream of the promoter, is a transcriptional regulatory element and stimulates BCL2 gene activity (6). The regulatory function of the mbr is closely related to SATB1 (7). However, the mechanism by which the mbr distal element executes its long-range regulatory function remains to be elucidated.
It is increasingly clear that genomic regulation by distal elements involves the formation of direct physical associations between distal elements and their target genes with the intervening chromatin being ‘looped out’ (8–14). In case of enhancers, chromosomal interaction appears to be specific and to require defined proteins to mediate association between particular sets of genomic elements (12,15–18). Many factors, including MAR binding proteins, transcriptional factors and RNA polymerase II, have been suggested for roles in the formation of chromatin loops (15,19–21). For instance, SATB1-mediated loop formation is found to be important for the expression of the cytokine genes, β-globin gene cluster and MHC I locus (15,22–24). GATA-1 and its cofactor FOG-1 are required for the physical interaction between β-globin locus control region (LCR) and β-globin promoter (20). SATB1 is primarily and highly expressed in thymocytes. It belongs to a class of transcription factors that function as a landing platform for several chromatin remodeling enzymes and hence regulates the epigenetic status in a large chromatin domain (25). Nevertheless, the connection of SATB1-mediated long-range regulation and expression of specific genes controlling apoptosis pathway has not been established. The mbr is known to be the binding site of SATB1 (26). The close relation of the mbr regulatory function and SATB1 revealed by our previous studies (7) implies an involvement of SATB1 in the long-range regulation of the BCL2 gene.
In the present work we focused on the two main issues: (i) whether the mbr distal element executes its long-range regulatory function by physically interacting with the BCL2 promoter through SATB1-mediated chromatin looping and (ii) what the biological significance of the mbr-BCL2 promoter interaction is. To address these questions we systematically investigated SATB1-mediated interaction between the mbr distal element and BCL2 promoter, the correlation of mbr-promoter interaction with epigenetic modifications of the promoter, in vivo and in vitro status of C/EBPβ and p300 binding, accessibility of critical transcriptional factor CREB to BCL2 promoter as well as transcription activity of the BCL2 gene in Jurkat cells using chromosome conformation capture (3C) methodology, chromatin immunoprecipitation (ChIP) and electrophoretic gel mobility shift assay (EMSA). We found that the mbr distal element physically interacted with the promoter that is 200 kb away, through SATB1-mediated chromatin looping. The physical interaction between the mbr and the promoter was required for the epigenetic modifications of the promoter, CREB binding and high expression of the BCL2 gene. Reduced mbr-promoter interaction by knockdown of SATB1 significantly decreased the transcriptional activity of the BCL2 gene and increased cell apoptosis rates. Inhibition of SATB1 cleavage with 10 μM Z-VEID-fmk, a specific inhibitor of caspase-6 inhibited disassembly of the SATB1-mediated chromatin loop and restored the BCL2 mRNA level in Jurkat cells in response to apoptotic stimulation. The effect of SATB1 cleavage on BCL2 expression was further confirmed by overexpression of mutant SATB1 that was resistant to caspase-6. Our study provides the first evidence that the SATB1-mediated long-range regulation played an important role in regulation of the BCL2 gene in response to cellular apoptosis.
MATERIALS AND METHODS
Cell culture and induction of apoptosis
Human T lymphoid cell line Jurkat is a generous gift from Dr Krontiris’ Laboratory at City of Hope National Medical Center in Los Angeles, USA. Jurkat cells were grown in RPMI 1640 medium supplemented with 10% FBS, 10 mM HEPES, 100 U penicillin per ml and 10 μg streptomycin per ml. The cells were incubated at 37°C in humidified atmosphere containing 95% air/5% CO2. For induction of apoptosis, Jurkat cells were treated with 5 μM camptothecin for 2 h and subsequently harvested. The vehicle control for camptothecin is the equal volume of DMSO. For protease inhibitor assay, Jurkat cells were preincubated with 10 μM Z-VEID-fmk for 30 min. Apoptosis was then induced by the addition of camptothecin.
ChIP assay
ChIP assays were performed using the ChIP assay kit essentially as described in the manufacturer (Upstate). Briefly, Jurkat cells (1 × 107) were fixed in RPMI 1640 medium containing 1% formaldehyde for 10 min at room temperature. After cell lysis, genomic DNA was sheared into 200–1000 bp fragments using Sonics VCX130 (SONICS). Sheared chromain was incubated with anti-SATB1 (a generous gift from Dr Krontiris’ Laboratory at City of Hope National Medical Center in Los Angeles, USA), anti-CREB (Santa Cruz), anti-Acetyl-H3 (Upstate), anti-Acetyl-H4 antibody (Upstate) anti-C/EBPβ (Santa Cruz), anti-p300 (Santa Cruz), anti-CBP (Santa Cruz)and IgG (Upstate) overnight at 4°C, respectively. NaCl was added to the ChIP samples for 4 h at 65°C to reverse the cross-links. To purify the immunoprecipitated DNA, RNase and proteinase K were added in order, followed by phenol–chloroform extraction, ethanol precipitation and resuspension of the DNA in distilled water. The input genomic DNA and the immunoprecipitated DNA was then amplified by PCR using specific primers listed in Supplementary Table S1. The PCR cycling parameters were as follows: 30 s at 95°C, 30 s at 56°C, 30 s at 72°C, for 32 cycles. The PCR products were subjected to gel electrophoresis, stained with ethidium bromide and analyzed on Molecular Imager Gel Doc XR System (Bio-Rad).
Quantification of ChIP assay
For quantitative analysis of ChIP products, real-time PCR (ABI PRISM 7000 system, Applied Biosystems) was carried out according to TOYOBO manufacturer’s instructions using SYBR Green real-time PCR Master Mix (TOYOBO). Primers designed for specifically amplifying SBS1, P1 and mbr of BCL2 were listed in Supplementary Table S1. Annealing temperatures were as follows: 63°C for SBS1 and mbr amplicons; 65°C for P1 amplicon. Standard curves for relative quantification were calculated using 1:2, 1:4, 1:8 and 1:16 dilutions of input samples. All PCR signals from immunoprecipitation samples were referred to their respective input standard curve to normalize differences in cell number and primer efficiency. Real-time PCR data were analyzed according to the methodology described in a recent report (27). ΔCt values were first calculated using the formula: ΔCt =Ct sample − Ct input, from which ΔΔCt (ΔCt experiment sample − ΔCt negative control) and fold difference [2(−ΔΔCt trememt)/2(−ΔΔCt control)] were derived.
Chromosome conformation capture
The principle of 3C assay is summarized in Supplementary Figure S1. The 3C protocol was adapted from Jiang et al. (2008) (28) and Hagege et al. (29) with modifications. Cross-linking was achieved by incubating 1 million cells in 2 ml medium containing 2% formaldehyde for 5 min at room temperature and stopped by adding glycine to 0.125 M. Cells were then lysed in 1.25 ml lysis buffer (10 mM Tris–HCl at pH 8.0, 10 mM NaCl, 0.2% NP-40) with freshly added protease inhibitors for 60 min at 4°C with rotation. The nuclei were suspended in 1.2× restriction buffer (Buffer 2 for SBS2/mbr or Buffer 3 for SBS1/mbr, NEB) + 0.3% SDS and incubated at 37°C for 1 h with shaking. The SDS was then sequestered by adding Triton X-100 to 1.8% and incubating at 37°C for another hour with shaking. Hundred units of the restriction enzyme (NEB) (AseI for SBS1-mbr, HindIII for SBS2/mbr) were added for a 22-h digestion. The reaction was stopped by adding SDS to 1.6% and incubating at 65°C for 20 min. About 2 μg of digested chromatin was diluted in 800 μl ligation buffer (NEB). Residual SDS was sequestered by adding Triton X-100 to 2% and incubating at 37°C for 1 h with shaking. The reaction was then cooled to 16°C and 2000 U of T4 DNA ligase (NEB) were added. After ligation overnight, the chromatin mixture was incubated with 100 μg/ml proteinase K at 65°C overnight to reverse crosslinks. RNA was removed by RNase A (0.5 μg/ml) treatment for 30 min at 37°C. The 3C sample was purified by phenol–chloroform extraction and then amplified by PCR using specific primers listed in the Supplementary Table S1. The PCR cycling parameters were as follows: 30 s at 95°C, 30 s at 56°C/59°C, 30 s at 72°C, for 35 cycles. PCR products from both AseI or HindIII digested crosslinked chromatin without ligation and non-crosslinked genomic DNA with or without ligation were used as negative controls. All PCR products were sequenced.
To normalize primer efficiency control PCR templates were generated by digestion and random ligation of bacterial artificial chromosomes containing BCL2 gene (clone CTD-2270P21, CTD-3241O8, Invitrogen) or PGK1 gene (CTD-2255G23, Invitrogen). A total of 5 μg of one BAC clone or equal molar amounts of two BAC clones were digested with AseI and then ligated at a high DNA concentration.
Quantitative real-time PCR was performed, in the presence of SYBR Green, with appropriate primers from purified DNA as well as the control template. To accurately determine the DNA concentration of a 3C sample, 20-dilutions of each 3C sample was first analyzed by real-time PCR using SBS1 ChIP primers and the DNA concentration was calculated according to the standard curve that was established using reference genomic DNA with known concentrations. Samples were then subsequently adjusted to a concentration of 50 ng/μl. For each primer pair successive 4-fold (PGK1) or 2-fold (BCL2) dilutions of the random ligation standard were used to make a calibration curve for determining a relative quantity of the corresponding ligation product in a 3C sample. The ligation efficiency between the samples was corrected by the interaction between two AseI fragments within the ubiquitously expressed PGK1 locus that had been checked to be transcribed stably in our experiment systems (Supplementary Tables S2, S3 and Figure S2).
Electrophoretic mobility shift assays
Nuclear extracts were prepared using NE-PER nuclear and cytoplasmic extraction Reagents (Pierce) following the manufacturer’s instructions. In brief, Jurkat cells were harvested and washed with cold PBS. The cell pellet was suspended in CRE I buffer and incubated on ice for 10 min. Following incubation with CRE II solution, the preparation was centrifuged at 12 000g for 5 min. The pellet was treated with nuclear extraction reagent (NER) with vortexing for 15 s every 10 min, for a total of 40 min. After centrifugation at 12 000g for 10 min, the remaining supernatant was the nuclear extract. The protein concentrations were measured using a Bio-Rad protein assay.
The electrophoretic mobility shift assays (EMSA) was performed using a gel shift assay kit following the manufacturer’s instructions (Promega Corp.). Oligonucleotide probes were labeled with [γ-32P] ATP by T4-polynucleotide kinase (New England Biolabs Ltd, Beijing, China). A total of 10 μg of Jurkat nuclear extracts were incubated with 0.05 pmol of labeled probes in the gel shift binding buffer for 20 min at room temperature. The samples were separated on a 4% polyacrylamide gel. Signals were recorded on X-ray film. The sequences of probes were synthesized: 42 mbr-F, 5′-TATTTTATGAAAGGTTTACATTGTCAAAGTGATGAATATGGA-3′; 42 mbr-R, 5′-TCCATATTCATCACTTTGACAATGTAAACCTTTCATAAAATA-3′; SBS1-F: 5′-GATTCCATGTTTATTAGATTCCATTTTAAAG-3′; SBS1-R: 5′-CTTTAAAATGGAATCTAATAAACATGGAATC-3′; the non-specific competitive probe, SP1-F, 5′-ATTCGATCGGGGCGGGGCGAGC-3′ and SP1-R, 5′-GCTCGCCCCGCCCCGATCGAAT-3′. For competition experiments, either specific or non-specific oligonucleotide competitor was added to the binding mixture 10 min before addition of the labeled probe. For supershift experiments, anti-C/EBPβ (Santa Cruz), anti-p300 (Santa Cruz) and anti-SATB1 (Santa Cruz) were preincubated with the nuclear extracts in the binding reaction buffer for 40 min at room temperature, respectively.
Isolation of genomic DNA and methylation-specific PCR
Genomic DNA was isolated using AxyPrep™ Multisource Genomic DNA Miniprep kit (Axygen). Then, 1 μg of genomic DNA from each sample was modified by sodium bisulfite with the CpGenome™ DNA Modification Kit (Chemicon). The primers for amplification of methylated DNA were BCL2-MF and BCL2-MR; the primers for amplification of unmethylated DNA were BCL2-UF and BCL2-UR. All methylation-specific PCR (MSP) were performed for 35 cycles with Hot Star Taq DNA Polymerase (Qiagen). The final PCR products of MSP were randomly selected for sequencing (Invitrogen). CpGenome™ universal unmethylated DNA and methylated DNA (Chemicon) were used as controls for MSP analysis. The primer sequences used in this study were listed in Supplementary Table S1.
Quantitative methylation specific real-time PCR
Methylation analysis was performed with real-time PCR similar to the Methylight technique as described previously (30). The bisulfite-treated genomic DNA obtained was subjected to real-time PCR analysis. PCR conditions consisted of an initial denaturation for 1 min at 95°C followed by 40 cycles of denaturation for 15 s at 95°C, following annealing for 1 min at 63°C. PCR analysis of each sample was repeated three times. Data were analyzed through the comparative threshold cycle (Ct) method. In order to normalize the input bisulfite-converted DNA, control reactions were carried out for non-CpG sites of control gene β-Actin, which has been routinely used in previous reports as a measure of input bisulfite-treated DNA levels (31–33). The β-Actin primers used for quantitative methylation specific real-time PCR (QMSP) are shown in Supplementary Table S1. For each analysis, the average Ct (Ct BCL2 − Ct Actin) of three experiments was calculated. Then the mean fold change (2−ΔΔCt) of BCL2 unmethylation status and variations in Jurkat cells were determined during all treatments.
Knock down SATB1 by RNAi in Jurkat cells
SATB1-specific shRNA sequences were synthesized according to the one used in Han et al. (34) and inserted into the pGCsi-H1/Neo/GFP/siNEGative vector (Genscript), which coexpresses GFP to allow identification of transfection efficiency. The SATB1 shRNA sequence was: SATB1-shRNA 5′-GTCCACCTTGTCTTCTCTC-3′. The non-specific shRNA sequence was: control-shRNA 5′-ACGTGACACGTTCGGAGAA-3′ (7). Jurkat cells were transiently transfected with SATB1 RNAi plasmids or control plasmids using an electroporator. The extent of shRNA mediated inhibition of SATB1 and its effect on BCL2 expression were evaluated by western blot analysis and RT–RCR respectively.
Western blot analysis
Whole cell extracts were prepared from cells (treated as described above) using lysis buffer (50 mM Tris, pH 7.4, 0.5% NP-40 and 0.01% SDS) containing protease inhibitors. Total protein (20 μg) was boiled for 5 min in loading buffer, chilled on ice and then separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels. Subsequent to transfer onto PVDF membranes (Bio-Rad), non-specific protein interactions were blocked by incubation in 5% non-fat dry milk in TST buffer (50 mM Tris–HCl, 150 mM NaCl, 0.05% Tween-20, pH 7.6) at 4°C for 1 h. Membranes were then incubated at 4°C overnight with anti-SATB1, anti-CREB or anti-β-Actin antibody (Sigma) in fresh blocking buffer. Horseradish peroxide–conjugated individual secondary antibody (R&D) was added for 1 h at room temperature. The blot was developed with ECL reagent (Amersham Biosciences). Prestained markers (NEB) were used as internal molecular weight standards.
RNA isolation and RT–RCR
Total RNA was isolated with Trizol reagent (Invitrogen) according to the manufacturer’s protocol. RNA integrity was assessed by visualizing the ribosomal bands on a 1% agarose gel. Finally, cDNA was synthesized from total RNA (1 μg) using AMV reverse transcriptase according to the manufacturer’s instructions (TOYOBO) and oligo(dT)15 as the primer. The reactions were incubated at 42°C for 60 min and then stored at −20°C prior to use. The real-time PCR amplification conditions were 50°C for 2 min, 95°C for 1 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 63°C for 1 min.
Generation of caspase-6 resistant mutant SATB1 expression vector SATB1-D254A
To prepare the mutant SATB1 expression plasmid SATB1-D254A that is resistant to caspase-6 cleavage, aspartate at position 254 in the human SATB1 primary sequence from SATB1 overexpression plasmid pEGFP-C1-SATB1 (generous gifts from Dr Krontiris laboratory) was replaced with alanine using the QuikChange® Site-Directed Mutagenesis Kit (Stratagene). The primer used for mutagenesis is as follows with the mutated bases in boldface: mut-F, 5′-GATATGATGGTTGAAATGGCTAGTCTTTCTGAGCTATC-3′. The mutant plasmid sequence was confirmed by sequencing.
Assay for apoptosis
Apoptosis was estimated by using an Apoptosis Detection Kit (Keygentech) according to the manufacturer’s instruction. Briefly, cells were first resuspended in binding buffer. Annexin V-EGFP and propidium iodide (PI) were then added and incubated at room temperature for 15 min in the dark, followed by assay on FACScan (Becton Dickinson). The percentage of apoptosis was computed using CellQuest software (Becton Dickinson).
RESULTS
SATB1 binds to BCL2 promoter and mbr
Our previous work has demonstrated that the long-range regulatory function of the mbr is closely related to SATB1 that is known to bind to the mbr (6,7). To investigate whether the regulatory function of the mbr is dependent on SATB1-mediated modulation of chromatin structure within the BCL2 gene, we first searched for the potential binding sites of SATB1 in the BCL2 promoter region using Genomatix Software (http://www.genomatix.de/index.html). SATB1 prefers sequences that have the characteristic ‘ATC sequence context’, which is enriched in stretches of DNA sequences containing a mixture of adenine, thymidine and cytosine (but not guanine), on one strand (35), although recent studies have suggested a consensus element that may not follow this rule (36,37).
BCL2 has two promoters, P1 and P2. P1 is located 1386 to 1423 bp upstream of the translational start site and is the major transcriptional promoter; while P2, located 1.3 kb downstream from P1, only has primary functions in specific tissues, such as t(14;18) lymphoma cells and neuronal cells (38,39). Therefore, in this study we focused on P1. Using bioinformatic analysis, we found three sequences, named from SBS1 to SBS3 that might possibly bind to SATB1 in the 5.1-kb region upstream of the P1 promoter.
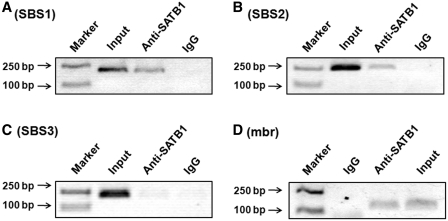
To determine whether SATB1 can bind to these three sequences in vivo, we performed ChIP assays using an anti-SATB1 antibody. SBS1 and SBS2 sequences located −4.1 and −4.7 kb relative to the translational start site (Supplementary Figure S3) were found to be specifically immunoprecipitated with anti-SATB1 (Figure 1A and B), indicating that SATB1 binds to these sequences in vivo. The ChIP assays on the SBS3 sequence did not reveal any binding of SATB1 (Figure 1C). ChIP assays targeting to the mbr were also performed and the binding of SATB1 to the mbr was confirmed in our experimental system (Figure 1D). The binding of SATB1 to both BCL2 promoter and mbr strongly implied that SATB1 might mediate an interaction between the mbr element and the promoter by modification of BCL2 chromatin structure.
Figure 1.
ChIP analysis of binding of SATB1 at the mbr and the BCL2 promoter region in vivo. The ChIP experiments were performed as described in the ‘Materials and Methods’ section. The results showed that SATB1 bound to SBS1 (A), SBS2 (B) and mbr (D), respectively, but not to SBS3 (C). The input represented 1% of total chromatin used in immunoprecipitation. Non-specific IgG was used as a negative control. The PCR products were visualized by ethidium bromide staining of a 1.5% agarose gel.
The mbr physically interacted with the BCL2 promoter in Jurkat cells
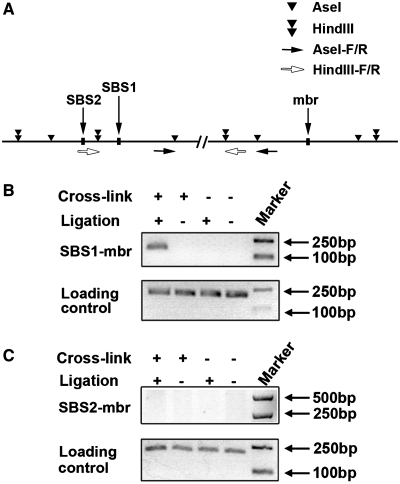
To investigate whether the mbr regulates BCL2 transcriptional activity over the long chromatin distance by physically interacting with the promoter, 3C technique was used to analyze the spatial organization of BCL2 in Jurkat cells. The principle of 3C assay is summarized in Supplementary Figure S1. For 3C analysis, formaldehyde is used to crosslink protein–DNA in intact nuclei. The crosslinked chromatin was then digested by a restriction enzyme, followed by ligation. If there is apposition between a remote regulatory sequence and a promoter, new hybrid fragments containing these two elements are generated; and carefully designed PCR reactions can be used to detect and quantify these new combined fragments (Figure 2A and Supplementary Figure S1). In our experiments AseI or HindIII was used to digest nuclei to evaluate the interaction between the mbr and SBS1 or SBS2. PCR bands resulting from the ligation products indicated that the mbr was linked with SBS1 (Figure 2B), but not with SBS2 (Figure 2C). DNA sequencing further confirmed that the ligation-dependent PCR products were derived from ligation of two distal chromatin fragments (the mbr and SBS1) within the BCL2 gene (Supplementary Figure S4). PCR was also performed using the BAC clone to ensure the pair of primers for SBS/mbr worked on the random ligation control (Supplementary Figure S5). Negative control samples either from non-crosslinked chromatin or from cross-linked chromatin that was digested without subsequent ligation did not generate any PCR products (Figure 2B and C). These results clearly indicate that the mbr and promoter (SBS1) of the BCL2 gene interact with each other in close proximity via chromatin looping.
Figure 2.
3C Analysis of the interaction between the mbr and the BCL2 promoter region in vivo. The scheme of BCL2 gene with the positions of restriction sites and positions of primers was indicated (A). The 3C experiments were performed as described in the ‘Materials and Methods’ section. Briefly, formaldehyde was used to crosslink protein–DNA interactions in intact nuclei. The crosslinked chromatin was then digested by AseI (B) or HindIII (C), followed by ligation. Physical interaction between mbr and SBS1 or SBS2 was then determined by specific PCRs that detected hybrid fragments containing either mbr/SBS1 sequences or mbr/SBS2 sequences. 3C data demonstrated that the mbr specifically interacted with SBS1 (B, up panel), but not with SBS2 (C, up panel). PCR products from AseI (B) or HindIII (C) digested crosslinked chromatin without ligation and non-crosslinked genomic DNA with or without ligation were used as negative controls. The bands shown in the bottom panels of B and C represented the PCR products from genomic DNA that was not cut by any restriction enzyme, which were used as the loading control.
SATB1 was required for the mbr-promoter interaction and BCL2 transcription
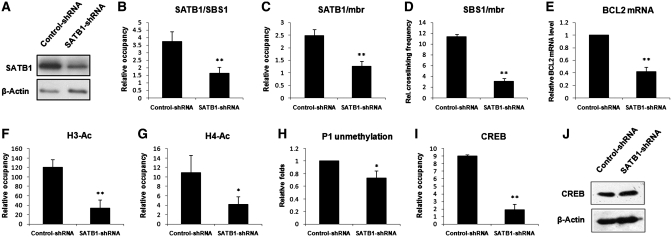
To address the question of whether SATB1 is required for the chromatin loop organization within the BCL2 gene, we determined the correlation between the binding of SATB1 to the two distal elements and the SBS1-mbr interaction. The level of SATB1 in Jurkat cells was knocked down with plasmids expressing short hairpin RNAs (shRNA). Western blot analysis confirmed that shRNA targeted to SATB1 efficiently reduced the expression level of SATB1 (Figure 3A). Quantitative ChIP assays revealed that knockdown of SATB1 significantly reduced the occupation of SATB1 on SBS1 and mbr (Figure 3B and C) and simultaneously decreased the frequency of interaction between SBS1 and mbr as indicated by quantitative 3C assay (Figure 3D). These observations clearly indicate that SATB1 is a critical mediator of long-distance interaction between the mbr and the BCL2 promoter.
Figure 3.
The Association of SATB1 with the chromatin-loop organization and transcription of BCL2 in Jurkat cells. (A) Western blot analysis of SATB1 in Jurkat cells transiently transfected with control-shRNA or SATB1-shRNA plasmids, respectively indicated that SATB1-shRNA effectively reduced the SATB1 level in the cells. (B) Quantitative ChIP results obtained from Jurkat cells that were transiently transfected with control-shRNA or SATB1-shRNA plasmids showed that knockdown of SATB1 expression significantly reduced the association of SATB1 with SBS1. (C) Quantitative ChIP results obtained from the same cells described above confirmed that knockdown of SATB1 expression also significantly reduced the association of SATB1 with the mbr. (D) Quantitative 3C assay was performed as described in ‘Materials and Methods’ section in parallel with the experiments in B and C. The relative crosslinking frequency between the fragments of SBS1 and mbr indicated that knockdown of SATB1 significantly suppressed the mbr-SBS1 (promter) interaction. (E) RT–PCR analysis of the BCL2 mRNA from Jurkat cells transfected with either control-shRNA or SATB1-shRNA. (F, G and I) The histone acetylation and CREB accessibility were analyzed with quantitative ChIP in Jurkat cells transiently transfected with control-shRNA or SATB1-shRNA plasmids using antibodies against acetylated histone H3 at K9/14 (F), acetylated histone H4 at K5/8/12/16 (G) and CREB (I). (H) The methylation level of the BCL2 P1 region was evaluated in the Jurkat cells described above using QMSP. (J) Western blot analysis of CREB in Jurkat cells transiently transfected with control-shRNA or SATB1-shRNA plasmids. The statistical differences were calculated using t-test. ‘*’ represents P < 0.05 and ‘**’ represents P < 0.01. The error bars represent standard deviation (n = 3).
We then determined whether SATB1-mediated chromatin looping was involved in transcriptional activity of the BCL2 gene by examining the correlation of BCL2 mRNA level and mbr-promoter interaction with RT–PCR and quantitative 3C assay. Our data showed that a reduction of the interaction between SBS1 and mbr induced by knockdown of SATB1 was significantly correlated with a decrease in the BCL2 mRNA level (Figure 3E), suggesting that SATB1-mediated mbr-promoter interaction was required for transcriptional activity of the gene.
The SATB1-mediated mbr-promoter interaction was required for epigenetic modifications of the BCL2 P1 region
Active and silent gene expression states can be characterized by various epigenetic marks, including histone acetylation and DNA methylation. Acetylated histone H3 at K9/14 and H4 at K5/8/12/16 are normally marks for active chromatin (40,41). To evaluate the role of SATB1-mediated interaction between the mbr and promoter in epigenetic modification of BCL2, we compared the acetylation status of histones in the P1 region of the BCL2 gene in Jurkat cells in which SATB1 expression was knocked down with that in the control cells by quantitative ChIP assays using anti-H3 K9/14-Ac and anti-H4 K5/8/12/16-Ac antibodies in parallel with the experiments described in Figure 3A–E. As indicated in Figure 3F and G, the acetylation levels of histones H3 and H4 in the P1 region were dramatically reduced when SATB1 was silenced using shRNA.
The BCL2 P1 region is extremely GC rich. Bioinformatic analysis has identified a CpG island in the region that contains 147 CpG sites, spanning the region from −2434 to −1174 bp relative to the translation start site (42). Methylation status examined within this region was evaluated by quantitative methylation-sensitive PCR (QMSP). The primers were designed to produce an unmethylated region from −1552 to −1360 bp and a methylated region from −1549 to −1361 bp (Supplementary Table S1). All samples revealed unmethylated products, while no methylated products were detected in Jurkat cells that highly express SATB1. The unmethylation of the P1 region decreased in Jurkat cells when SATB1 was knocked down (Figure 3H). These data clearly demonstrated that SATB1-mediated chromatin looping was required for epigenetic modification in the P1 region.
The SATB1-mediated mbr-promoter interaction enhanced recruitment of the CREB transcription factor to P1
The cAMP response element (CRE) is one of the major positive cis-regulators for P1 activity (43). The CREB transcription factor can function through CRE to protect diverse cell types from apoptosis by up-regulating BCL2 (44). To further evaluate the role of SATB1-mediated special interaction between the mbr and the BCL2 promoter in regulation of BCL2 expression, the binding status of the CREB transcription factor at P1 was also examined relative to the chromatin looping in Jurkat cells. Again, cells in which SATB1 was knocked down were compared with the control cells. We found that silencing of SATB1 significantly suppressed CREB binding to P1 even though the level of CREB expression was not affected (Figure 3I and J), indicating that recruitment of CREB to P1 was primarily dependent on the SATB1-mediated epigenetic modification in the P1 region, particularly the mbr-promoter interaction.
C/EBPβ and p300 were associated with both the mbr and SBS1 in vivo
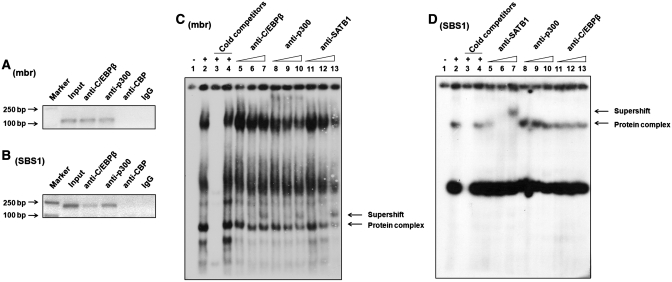
To identify the enzymes and proteins that are involved in epigenetic modification of the BCL2 promoter related to SATB1-mediated mbr-promoter interaction, we analyzed the mbr element and BCL2 promoter region using Genomatix Software (http://www.genomatix.de/index.html). A C/EBPβ consensus sequence was found on the 5′-boundary of the SATB1 binding site with partial overlapping with the SATB1 binding consensus in the mbr. However, no C/EBPβ consensus was identified at the SBS1. As CBP/p300 are well established coactivators for both C/EBPβ (45) and SATB1 (46,47) and possess HAT function (48,49), we first determined whether CBP/300 and C/EBPβ could bind to the mbr element by ChIP assay using anti-CBP, anti-p300 and anti-C/EBPβ antibodies, respectively. Our data showed that the mbr sequence was specifically immunoprecipitated with anti-p300 and anti-C/EBPβ, but not with anti-CBP antibody (Figure 4A), suggesting that p300 and C/EBPβ were specifically associated with the mbr and formed a complex with SATB1 in vivo.
Figure 4.
Analysis of the association of C/EBPβ and p300 with the mbr and the SBS1 sequence with ChIP and EMSA. (A) ChIP assay showed C/EBPβ (lane 3) and p300 (lane 4), but not CBP (lane 5), associated with the mbr in vivo. The PCR products amplified by specific primers for the mbr were visualized on 1.5% agarose gel. (B) ChIP analysis showed that C/EBPβ (lane 3) and p300 (lane 4), but not CBP (lane 5), associated with SBS1 in vivo. The DNA immunoprecipitated with different antibodies were amplified by specific primers for the SBS1. (C) EMSA demonstrated that C/EBPβ, p300 and SATB1 formed a protein complex at 42 mbr. Lane 1: the negative control without cell lysate. Lane 2: protein complexes formed at 42 mbr. Lane 3: 100-fold molar excess of the cold 42 mbr probe successfully competed with hot 42 mbr probe. Lane 4: 100-fold molar excess of the cold SP1 consensus sequence (the non-specific probe) failed to compete with the labeled 42 mbr that was bound by the protein complexes. Lane 5, 6, 7: the supershift experiment with 2, 6 and 10 μg anti-C/EBPβ antibody respectively. Lane 8, 9, 10: the supershift experiment with 2, 6 and 10 μg anti-p300 antibody respectively. Lane 11, 12, 13: the supershift experiment with 2, 6 and 10 μg anti-SATB1 antibody respectively. The arrow indicated the bands supershifted from the same DNA–protein complex with addition of three different antibodies. (D) EMSA demonstrated that SATB1, but not C/EBPβ or p300 binding to the SBS1 in vitro. Lane 1: the negative control without cell lysate. Lane 2: protein complexes formed at SBS1. Lane 3: the bands were successfully competed with 100-folds molar excess of the cold SBS1 probe. Lane 4: 100-folds molar excess of cold SP1 consensus sequence failed to compete with the SBS1 to be bound by the protein complexes. Lane 5, 6, 7: the supershift experiment with 2, 6 and 10 μg anti-SATB1 antibody respectively. Lane 8, 9, 10: the supershift experiment with 2, 6 and 10 μg anti-p300 antibody respectively. Lane 11, 12, 13: the supershift experiment with 2, 6 and 10 μg anti-C/EBPβ antibody respectively. As indicated by the arrow, DNA–protein formation was inhibited and supershifted by addition of anti-SATB1 antibody, while no blocked or supershifted band was observed with addition of anti-C/EBPβ or anti-p300 antibodies.
We further performed ChIP assay to examine whether p300 and C/EBPβ associate with SBS1 that contains no C/EBPβ binding consensus. The experimental data showed that SBS1 was specifically immunoprecipitated either by C/EBPβ or by p300 (Figure 4B), suggesting these two proteins could be recruited to SBS1 by SATB1 to form a complex in vivo.
C/EBPβ and p300 were associated with the mbr but not with the SBS1 in vitro
To evaluate the association of C/EBPβ and p300 with the mbr and SBS1 in vitro, in which no chromatin loop structure exists, EMSA experiment was performed using a 42-bp sequence from the mbr (42 mbr) as a probe that containing both the SATB1 binding site and the predicted C/EBPβ binding consensus. Several bands were formed when Jurkat nuclear extract was incubated with 32P-labeled 42 mbr probe (Figure 4C, lane 2). The bands were successfully competed by 100-fold molar excess of unlabeled 42 mbr cold probe, but not by cold SP1 control consensus sequences (Figure 4C, lanes 3 and 4), confirming the specific binding of the protein complexes to the 42 mbr probe. One of the protein complexes was supershifted by preincubating the nuclear extract with increasing amount (2, 6 and 10 μg) of anti-C/EBPβ and anti-p300, respectively (Figure 4C, lanes 5–10), indicating that C/EBPβ and p300 bind to the 42-bp mbr region in vitro. Increasing amount of anti-SATB1 (2, 6 and 10 μg) was added to the gel shift reactions as a positive control (Figure 4C, lanes 11–13). Interestingly, the supershifted bands resulted from addition of anti-C/EBPβ, anti-p300 and anti-SATB1 occurred at the same position above the fourth protein complex (indicated, Figure 4C, lanes 7, 10 and 13), implying that C/EBPβ, p300 and SATB1 can form the same protein complex that binds to the mbr element in vitro.
We then checked whether C/EBPβ, p300 and SATB1 can form a protein complex at the SBS1 by EMSA using SBS1 consensus as a probe. With a competition experiment, we identified two protein complexes formed at SBS1 (Figure 4D, lanes 1–4). The complex 1 was supershifted when increasing amount of anti-SATB1 (2, 6 and 10 μg) was added to the binding reaction system (Figure 4D, lanes 5–7). In contrast no supershifted band was observed with addition of either anti-p300 (Figure 4D, lanes 8–10) or anti-C/EBPβ (Figure 4D, lanes 11–13) antibody. These results indicate that C/EBPβ and p300 are not associated with the SBS1 in vitro, although SATB1 binds to the SBS1 under the same condition.
SATB1 was required for occupancy of C/EBPβ and p300 at SBS1 in Jurkat cells
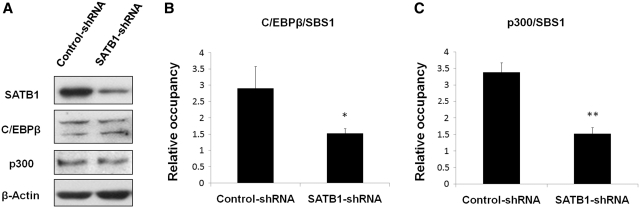
To further investigate whether C/EBPβ and p300 could be recruited to SBS1 by SATB1, ChIP experiments were performed using SATB1 knockdown cells to examine if it affected the occupancy of C/EBPβ and p300 at SBS1. Specific SATB1 shRNA plasmids were transiently transfected into Jurkat cells as described above. Western blot analysis showed that knockdown of SATB1 by RNAi did not affect expression of C/EBPβ and p300 significantly (Figure 5A). However, quantitative ChIP assays revealed that the occupancy of C/EBPβ and p300 at SBS1 was significantly decreased when SATB1 was knocked down (Figure 5B and C). These data demonstrated that SATB1 was directly involved in recruitment of C/EBPβ and p300 at SBS1 locus.
Figure 5.
Knockdown of SATB1 decreased the occupancy of C/EBPβ and p300 at SBS1 in Jurkat cells. Jurkat cells were transiently transfected with SATB1 RNAi plasmids or control plasmids using an electroporator. Western blot showed that knockdown of SATB1 did not affect expression levels of C/EBPβ and p300 significantly (A). Real-time PCR analysis demonstrated that knockdown of SATB1 significantly reduced the occupancy of C/EBPβ (B) and p300 (C) at SBS1. The statistical differences were calculated using t-test. *P < 0.05 and **P < 0.01. The error bars represent standard deviation (n=3).
The SATB1-mediated mbr-promoter interaction regulated response of the BCL2 gene to apoptosis stimulation
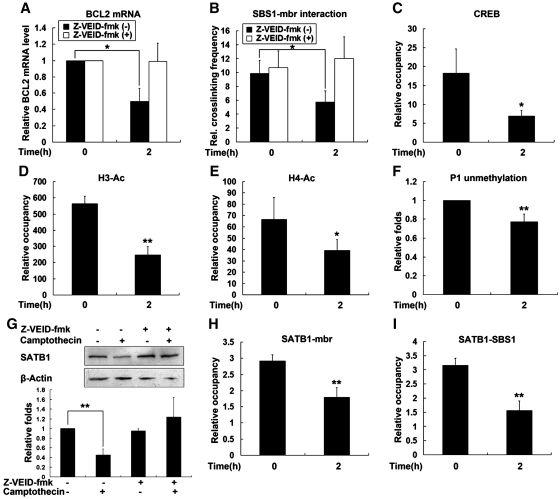
BCL2 is a key regulator of apoptosis. To address the issue whether SATB1-mediated interaction between mbr and the promoter of the BCL2 is directly involved in response of the gene to apoptosis stimulation, we analyzed the change in chromatin looping, histone acetylation, DNA methylation, CREB recruitment and transcription activity of the gene in parallel in Jurkat cells treated with camptothecin. Cells were incubated with 5 μM camptothecin and harvested after 2 h, when early apoptosis occurred (Supplementary Figure S6). RT–PCR results showed that camptothecin treatment reduced the BCL2 mRNA levels by 50% (Figure 6A). Correspondingly, quantitative 3C assays revealed that the frequency of interaction between SBS1 and the mbr (SATB1-mediated chromatin loop) was reduced (Figure 6B) in camptothecin-treated cells. The diminished BCL2 chromatin looping was accompanied by reductions both in CREB recruitment and acetylation of histone H3 and H4 in the P1 region of BCL2 (Figure 6C–E). Unmethylation of this region slightly decreased in the treated cells (Figure 6F). Corresponding to these changes, the level of SATB1 was clearly decreased in the camptothecin-treated cells as revealed by western blot analysis (Figure 6G). Quantitative ChIP assays further confirmed that less SATB1 occupied the mbr and P1 region (Figure 6H and I), which could explain why SATB1-mediated chromatin looping was diminished upon apoptosis induction. It should be pointed out that genomic integrity was maintained under our experimental conditions, which was confirmed by the slight increase in the frequency of the chromatin loop formed between the mbr and the promoter of the Noxa gene, a pro-apoptotic gene that located 3.4 Mb downstream of the BCL2 gene (Supplementary Figure S7). These data suggested that the SATB1-mediated mbr-promoter interaction was directly involved in regulation of the BCL2 gene in cellular apoptosis response.
Figure 6.
The association between SATB1-mediated chromatin looping and response of the BCL2 gene to apoptosis stimulation. For induction of apoptosis, Jurkat cells were treated with 5 μM camptothecin for 2 h and subsequently harvested. The vehicle control for camptothecin is the equal volume of DMSO. For protease inhibition assay, Jurkat cells were preincubated with 10 μM Z-VEID-fmk for 30 min and apoptosis was then induced by addition of camptothecin. (A) RT–PCR analysis of BCL2 mRNA from Jurkat cells described above. (B) Relative crosslinking frequencies between fixed fragments of SBS1 and mbr were determined by 3C assay in cells described above. (C–E) Quantitative ChIP results from Jurkat cells treated with camptothecin and the control, using antibodies against CREB (C), acetylated histone H3 at K9/14 (D) and acetylated histone H4 at K5/8/12/16 (E). (F) The methylation level of BCL2 gene P1 region was evaluated by examining the unmethylation status of this region using QMSP. (G) Western blot analysis of SATB1 from Jurkat cells treated with camptothecin or not. (H and I) Quantitative ChIP results from Jurkat cells with camptothecin treatment or not, using antibodies against SATB1. The statistical differences between the treatment groups were calculated using t-test. ‘*’ represents P < 0.05 and ‘**’ represents P < 0.01.The error bars represent standard deviation (n = 3).
SATB1 is known to be the target of caspase-6 and is degraded by this enzyme during early cell apoptosis. To confirm the functional role of SATB1-mediated chromatin loop in cell apoptosis, we further examined the effect of inhibition of SATB1 degradation on the mbr-promoter interaction and the BCL2 transcriptional activity in camptothecin-treated cells. Jurkat cells were pretreated for 30 min with 10 μM Z-VEID-fmk, an inhibitor of the caspase 6-like protease that specifically cleaves SATB1 during early apoptosis (50), prior to the addition of camptothecin. We found that pretreatment of cells with 10 μM Z-VEID-fmk completely inhibited camptothecin-induced cleavage of SATB1 (Figure 6G). Consequently, the decrease in BCL2 mRNA levels and the disassembly of higher-order chromatin structure were reversed in these cells (Figure 6A and B). As expected, pretreatment with Z-VEID-fmk also decreased the percentage of apoptotic cells (Supplementary Figure S6).
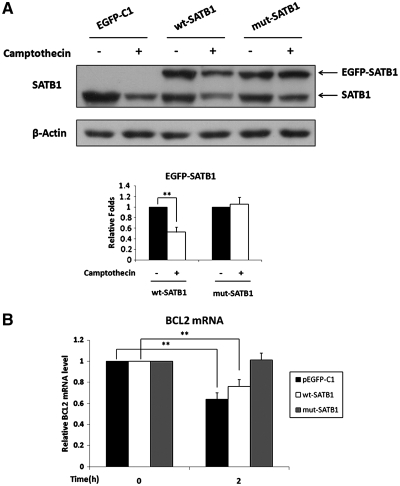
The effects caused by inhibition of SATB1 degradation on the BCL2 activity were further confirmed by the experiment using mutant SATB1 expression vector that was resistant to caspase-6 cleavage. In this experiment, Jurkat cells were transfected with a wild-type SATB1 expression vector (EGFP-SATB1), mutant SATB1 expression vector (EGFP-SATB1-D254A) and pEGFP-C1 control vector, respectively, before treatment with camptothecin. As shown in Figure 7, overexpression of the mutant undegradable SATB1 almost completely restored BCL2 mRNA levels in Jurkat cells treated by camptothecin for two hours, while overexpression of the wild-type SATB1 had only slight effect on BCL2 mRNA level under the same condition (Figure 7B).
Figure 7.
Mutation of SATB1 cleavability restored BCL2 transcriptional activity upon apoptosis stimulation. Jurkat cells were transiently transfected with the wild-type SATB1 expression vector (pEGFP-C1-SATB1), mutant SATB1 expression vector (SATB1-D254A, mutated at caspase cleavage site) and pEGFP-C1 control vector, respectively. Twenty-four hours after transfection, cells were treated with 5 μM camptothecin for 2 h to induce cell apoptosis. (A) Western blot analysis of SATB1 from Jurkat cells described above and quantitative analysis. The results confirmed that mutant SATB1 was un-degradable and wild-type SATB1 could be degraded in Jurkat cells treated with camptothecin. (B) RT–PCR analysis showed that camptothecin treatment reduced BCL2 mRNA level. Overexpression of mutant SATB1 completely restored the BCL2 expression, while overexpression of wild-type SATB1 only partially restored BCL2 expression. The statistical differences were calculated using t-test. ‘**’ represents P < 0.01. The error bars represent standard deviation (n = 3).
DISCUSSION
Regulation of BCL2 gene is of a great interest, given its fundamental importance for cell fate. In our previous work, we identified a new distal regulatory element, the mbr that is located within 3′-UTR of the BCL2 gene. The mbr element significantly enhances the transcriptional activity of BCL2 promoter (6,7). In the present study we uncovered a novel mechanism by which the mbr distal element implemented its activity at the BCL2 promoter. We have demonstrated: (i) the mbr distal element physically interacted with the promoter that was 200 kb away through SATB1-mediated chromatin looping. The SATB1-mediated BCL2 chromatin loop was required for the epigenetic modification, CREB accessibility and high expression of BCL2 gene. (ii) The SATB1-mediated BCL2 chromatin loop played a critical role in regulation of BCL2 expression in cellular response to apoptotic stimulation.
SATB1 has been reported to bind an AT-rich region immediately downstream of the mbr (26). Our present study identified a new SATB1 binding site, SBS1, that was located 4.1 kb relative to the translational start site of the BCL2 gene by bioinformatic analysis and ChIP assays. Further evaluation by quantitative ChIP and 3C assays was confirmed that SATB1 directly bound to the SBS1 and the distal mbr element with similar dynamics of assembly. The SATB1 binding to the two elements was required for the BCL2 chromatin loop formation, as knockdown of SATB1 expression by RNAi significantly decreased SATB1 binding and chromatin looping in Jurkat cells, which was accompanied by reductions in histone acetylation and the BCL2 transcriptional activity. These data lead us to propose that SATB1 tethers the mbr distal element and the BCL2 promoter together and mediates the long-range regulatory function of the mbr and thus is a critical regulator of BCL2 expression. Our observation that inhibiting SATB1 degradation with caspase-6 inhibitor reversed disassembly of the BCL2 chromatin loop further supports this idea. SATB1 may not be the only tie for the mbr-promoter interaction. However, it is the only linkage for this interaction in Jurkat cells of expressing high level of SATB1 so far. It is important to identify more components present in the protein complexes involved in the mbr-promoter interaction. Such studies are underway in our laboratory.
SATB1 belongs to a class of transcription factors that recruit chromatin remodeling complexes to bound sites and thereby regulate gene expressions over long distance (51). The function of SATB1 in gene regulation (up- or down-regulation) largely depends on the proteins it recruits, including histone deacetylases and acetyltransferase (44,51). P300 is a histone acetyltransferase that regulates transcription via chromatin remodeling (52). It is also known to be a co-activator for both C/EBPβ (45) and SATB1 (46,47). With ChIP assay we demonstrate that C/EBPβ and p300 form the complex with SATB1 at the mbr and the SBS1 (promoter) in vivo, where chromatin loops are allowed to form. However, EMSA assay found that C/EBPβ and p300 only formed the complex with SATB1 at the mbr, but not at the SBS1 in vitro, where no chromatin loop was allowed to form. The EMSA experimental data were consistent with the bioinformatic analysis results. A C/EBPβ consensus sequence was found on the 5′-boundary of the SATB1 binding site with partially overlapping with the SATB1 binding consensus in the mbr, but no C/EBPβ consensus was identified at the SBS1. The discrepancy in binding patterns of C/EBPβ and p300 in vitro and in vivo had two implications: (i) C/EBPβ and p300 were originally recruited to the mbr distal element and were brought to the SBS1 (promoter) through SATB1-mediated chromatin looping, which resulted in the increase in histone acetylation of the BCL2 promoter. This deduction was supported not only by the positive correlation of SATB1-dependent mbr-promote interaction with histone acetylation and DNA hypomethylation of the BCL2 promoter demonstrated by our quantitative ChIP and 3C assays (Figures 3, 5 and 6), but also by our observation that knockdown of SATB1 significantly decreased the occupancy of C/EBPβ and p300 at SBS1 (Figure 5); (ii) The recruitment of p300 to SATB1 targeted genomic site, such as the mbr and SBS1, might need the help of other proteins, such as C/EBPβ. From this point of view, it is not too surprising to see why SATB1-mediated chromatin loop (the mbr-promoter interaction) was required for the epigenetic modifications and maximal transcriptional activity of BCL2 promoter.
Emerging evidence strongly suggests that histone modifications set the state for DNA methylation (53). When histone is deacetylated, the chromatin remodeling complex binds and alters the chromatin structure or nucleosome positioning in such a way as to directly facilitate access of the DNMT to the nucleosomal DNA. The region is then methylated which locks the chromatin in a silent mode (54). In this study, the positive correlation of histone acetylation (histone H3 at K9/14 and H4 at K5/8/12/16) with DNA demethylation of the BCL2 promoter was confirmed in Jurkat cells where SATB1 was decreased either by RNAi or by apoptosis-induced cleavage. It is likely that alteration of histone acetylation induced by SATB1-mediated chromatin loop affects the methylation status of the BCL2 promoter by changing the chromatin structure and blocking the access of the DNMT to the nucleosomal DNA. Further investigation is required to prove this proposal.
Our present work is also of great significance in view of the functional role of SATB1-mediated chromatin loop in BCL2 regulation in cellular response to apoptotic stimulation. SATB1 is known to be the target of caspase-6 in cell apoptosis (55). During early Jurkat T-cell apoptosis, SATB1 is first removed from the bases of chromatin loop domains by caspase-6 cleavage, and that these domains are cleaved and digested further, which eventually disassemble higher-order chromatin structure and lead to nuclear degradation (50). To address the biological significance of SATB1-mediated BCL2 chromatin loop in cell apoptosis, the correlation between SATB1 cleavage and the long-range regulation of BCL2 during early apoptosis of Jurkat cells was carefully investigated under the experimental conditions when the integrity of genomic DNA was maintained. We demonstrated here that SATB1 cleavage beginning at a very early stage of apoptosis reduced the BCL2 chromatin loop structure by modulating the association of SATB1 with the mbr and the promoter, and consequently downregulated BCL2 transcriptional activity. Importantly, the downregulation of the BCL2 gene induced by SATB1 cleavage occurred before the genomic DNA was degraded. The integrity of the genomic DNA was proved by the presence of a 3.4-Mb intact chromatin loop formed between BCL2 and Noxa gene confirmed by 3C assay under the same experimental conditions. Inhibition of SATB1 cleavage by treatment of cells with caspase-6 inhibitor inhibited disassembly of the SATB1-mediated chromatin loop and restored BCL2 expression level. Additionally, overexpression of mutant SATB1 that was resistant to caspase-6 cleavage completely reversed the apoptosis-induced reduction of BCL2 transcription. These data strongly support the notion that the SATB1-mediated long-range interaction between the mbr and BCL2 promoter played a critical role in regulation of the BCL2 transcriptional activity in cellular response to apoptotic stimulation. An implication derived from our data is that SATB1-mediated chromatin conformation changes might be involved in coordination of genes controlling apoptosis pathway, since SATB1 has been considered to be a global organizer of gene expression in T lymphocytes (51). Identification of more BCL2 family members that are subjected to SATB1-mediated long-range regulation and investigation of their transcriptional correlation will provide valuable information for understanding the mechanisms underlying coordinated regulation of apoptosis-responsive genes at the level of three dimensional chromatin structures.
All together, our present study reveals a novel mechanism by which BCL2 gene is regulated and provides a useful model for the investigation of the nature of SATB1-mediated long-range interaction in regulating genes controlling apoptosis pathway.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Natural Science Foundation of China (30772490); Major National Natural Science Foundation of China (90919051); Special Funds for Major State Basic Research Program of China (973 Program, 2006CB503908). Funding for open access charge: Major National Natural Science Foundation of China (90919051).
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Prof. H.J. Gross, Prof. Z.J. Zhao and Prof. P.S. Liu for their advice on writing this article.
REFERENCES
- 1.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 2.Deng X, Gao F, May WS., Jr Bcl2 retards G1/S cell cycle transition by regulating intracellular ROS. Blood. 2003;102:3179–3185. doi: 10.1182/blood-2003-04-1027. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Pantschenko AG, McCarthy MB, Gronowicz G. Bone-targeted overexpression of Bcl-2 increases osteoblast adhesion and differentiation and inhibits mineralization in vitro. Calcif. Tissue Int. 2007;80:111–122. doi: 10.1007/s00223-006-0168-2. [DOI] [PubMed] [Google Scholar]
- 4.Adams JM, Huang DC, Puthalakath H, Bouillet P, Vairo G, Moriishi K, Hausmann G, O’Reilly L, Newton K, Ogilvy S, et al. Control of apoptosis in hematopoietic cells by the Bcl-2 family of proteins. Cold Spring Harb. Symp. Quant. Biol. 1999;64:351–358. doi: 10.1101/sqb.1999.64.351. [DOI] [PubMed] [Google Scholar]
- 5.Hara T, Omura-Minamisawa M, Kang Y, Cheng C, Inoue T. Flavopiridol potentiates the cytotoxic effects of radiation in radioresistant tumor cells in which p53 is mutated or Bcl-2 is overexpressed. Int. J. Radiat. Oncol. Biol. Phys. 2008;71:1485–1495. doi: 10.1016/j.ijrobp.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Ma C, Han X, Durrin LK, Sun Y. The bcl-2 major breakpoint region (mbr) possesses transcriptional regulatory function. Gene. 2006;379:127–131. doi: 10.1016/j.gene.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Ma C, Zhang J, Durrin LK, Lv J, Zhu D, Han X, Sun Y. The BCL2 major breakpoint region (mbr) regulates gene expression. Oncogene. 2007;26:2649–2657. doi: 10.1038/sj.onc.1210069. [DOI] [PubMed] [Google Scholar]
- 8.Gavrilov AA, Razin SV. Spatial configuration of the chicken alpha-globin gene domain: immature and active chromatin hubs. Nucleic Acids Res. 2008;36:4629–4640. doi: 10.1093/nar/gkn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berezney R, Mortillaro MJ, Ma H, Wei X, Samarabandu J. The nuclear matrix: a structural milieu for genomic function. Int. Rev. Cytol. 1995;162A:1–65. doi: 10.1016/s0074-7696(08)61228-0. [DOI] [PubMed] [Google Scholar]
- 10.Nickerson J. Experimental observations of a nuclear matrix. J. Cell. Sci. 2001;114:463–474. doi: 10.1242/jcs.114.3.463. [DOI] [PubMed] [Google Scholar]
- 11.Ottaviani D, Lever E, Takousis P, Sheer D. Anchoring the genome. Genome Biol. 2008;9:201. doi: 10.1186/gb-2008-9-1-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nativio R, Wendt KS, Ito Y, Huddleston JE, Uribe-Lewis S, Woodfine K, Krueger C, Reik W, Peters JM, Murrell A. Cohesin is required for higher-order chromatin conformation at the imprinted IGF2-H19 locus. PLoS Genet. 2009;5:e1000739. doi: 10.1371/journal.pgen.1000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Haene B, Attanasio C, Beysen D, Dostie J, Lemire E, Bouchard P, Field M, Jones K, Lorenz B, Menten B, et al. Disease-causing 7.4 kb cis-regulatory deletion disrupting conserved non-coding sequences and their interaction with the FOXL2 promotor: implications for mutation screening. PLoS Genet. 2009;5:e1000522. doi: 10.1371/journal.pgen.1000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackledge NP, Ott CJ, Gillen AE, Harris A. An insulator element 3′ to the CFTR gene binds CTCF and reveals an active chromatin hub in primary cells. Nucleic Acids Res. 2009;37:1086–1094. doi: 10.1093/nar/gkn1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai S, Lee CC, Kohwi-Shigematsu T. SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat. Genet. 2006;38:1278–1288. doi: 10.1038/ng1913. [DOI] [PubMed] [Google Scholar]
- 16.Barnett DH, Sheng S, Charn TH, Waheed A, Sly WS, Lin CY, Liu ET, Katzenellenbogen BS. Estrogen receptor regulation of carbonic anhydrase XII through a distal enhancer in breast cancer. Cancer Res. 2008;68:3505–3515. doi: 10.1158/0008-5472.CAN-07-6151. [DOI] [PubMed] [Google Scholar]
- 17.Choi YS, Hur J, Jeong S. Beta-catenin binds to the downstream region and regulates the expression C-reactive protein gene. Nucleic Acids Res. 2007;35:5511–5519. doi: 10.1093/nar/gkm547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grass JA, Jing H, Kim SI, Martowicz ML, Pal S, Blobel GA, Bresnick EH. Distinct functions of dispersed GATA factor complexes at an endogenous gene locus. Mol. Cell. Biol. 2006;26:7056–7067. doi: 10.1128/MCB.01033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yochum GS, Sherrick CM, Macpartlin M, Goodman RH. A beta-catenin/TCF-coordinated chromatin loop at MYC integrates 5′ and 3′ Wnt responsive enhancers. Proc. Natl Acad. Sci. USA. 107:145–150. doi: 10.1073/pnas.0912294107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M, Weiss MJ, Dekker J, Blobel GA. Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol. Cell. 2005;17:453–462. doi: 10.1016/j.molcel.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Ma Z, Terada LS, Garrard WT. Divergent roles of RelA and c-Rel in establishing chromosomal loops upon activation of the Igkappa gene. J. Immunol. 2009;183:3819–3830. doi: 10.4049/jimmunol.0901781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Di LJ, Lv X, Zheng W, Xue Z, Guo ZC, Liu DP, Liang CC. Inter-MAR association contributes to transcriptionally active looping events in human beta-globin gene cluster. PLoS One. 2009;4:e4629. doi: 10.1371/journal.pone.0004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar PP, Bischof O, Purbey PK, Notani D, Urlaub H, Dejean A, Galande S. Functional interaction between PML and SATB1 regulates chromatin-loop architecture and transcription of the MHC class I locus. Nat. Cell Biol. 2007;9:45–56. doi: 10.1038/ncb1516. [DOI] [PubMed] [Google Scholar]
- 24.Notani D, Gottimukkala KP, Jayani RS, Limaye AS, Damle MV, Mehta S, Purbey PK, Joseph J, Galande S. Global regulator SATB1 recruits beta-catenin and regulates T(H)2 differentiation in Wnt-dependent manner. PLoS Biol. 8:e1000296. doi: 10.1371/journal.pbio.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai S, Han HJ, Kohwi-Shigematsu T. Tissue-specific nuclear architecture and gene expression regulated by SATB1. Nat. Genet. 2003;34:42–51. doi: 10.1038/ng1146. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnan M, Liu WM, DiCroce PA, Posner A, Zheng J, Kohwi-Shigematsu T, Krontiris TG. Modulated binding of SATB1, a matrix attachment region protein, to the AT-rich sequence flanking the major breakpoint region of BCL2. Mol. Cell Biol. 2000;20:868–877. doi: 10.1128/mcb.20.3.868-877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay A, Deplancke B, Walhout AJ, Tissenbaum HA. Chromatin immunoprecipitation (ChIP) coupled to detection by quantitative real-time PCR to study transcription factor binding to DNA in Caenorhabditis elegans. Nat. Protoc. 2008;3:698–709. doi: 10.1038/nprot.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Peterlin BM. Differential chromatin looping regulates CD4 expression in immature thymocytes. Mol. Cell. Biol. 2008;28:907–912. doi: 10.1128/MCB.00909-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagege H, Klous P, Braem C, Splinter E, Dekker J, Cathala G, de Laat W, Forne T. Quantitative analysis of chromosome conformation capture assays (3C-qPCR) Nat. Protoc. 2007;2:1722–1733. doi: 10.1038/nprot.2007.243. [DOI] [PubMed] [Google Scholar]
- 30.Eads CA, Danenberg KD, Kawakami K, Saltz LB, Blake C, Shibata D, Danenberg PV, Laird PW. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:E32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widschwendter M, Jiang G, Woods C, Muller HM, Fiegl H, Goebel G, Marth C, Muller-Holzner E, Zeimet AG, Laird PW, et al. DNA hypomethylation and ovarian cancer biology. Cancer Res. 2004;64:4472–4480. doi: 10.1158/0008-5472.CAN-04-0238. [DOI] [PubMed] [Google Scholar]
- 32.Widschwendter M, Siegmund KD, Muller HM, Fiegl H, Marth C, Muller-Holzner E, Jones PA, Laird PW. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004;64:3807–3813. doi: 10.1158/0008-5472.CAN-03-3852. [DOI] [PubMed] [Google Scholar]
- 33.Friedrich MG, Chandrasoma S, Siegmund KD, Weisenberger DJ, Cheng JC, Toma MI, Huland H, Jones PA, Liang G. Prognostic relevance of methylation markers in patients with non-muscle invasive bladder carcinoma. Eur. J. Cancer. 2005;41:2769–2778. doi: 10.1016/j.ejca.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 34.Han HJ, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 2008;452:187–193. doi: 10.1038/nature06781. [DOI] [PubMed] [Google Scholar]
- 35.Dickinson LA, Joh T, Kohwi Y, Kohwi-Shigematsu T. A tissue-specific MAR/SAR DNA-binding protein with unusual binding site recognition. Cell. 1992;70:631–645. doi: 10.1016/0092-8674(92)90432-c. [DOI] [PubMed] [Google Scholar]
- 36.Kumar PP, Mehta S, Purbey PK, Notani D, Jayani RS, Purohit HJ, Raje DV, Ravi DS, Bhonde RR, Mitra D, et al. SATB1-binding sequences and Alu-like motifs define a unique chromatin context in the vicinity of human immunodeficiency virus type 1 integration sites. J. Virol. 2007;81:5617–5627. doi: 10.1128/JVI.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purbey PK, Singh S, Kumar PP, Mehta S, Ganesh KN, Mitra D, Galande S. PDZ domain-mediated dimerization and homeodomain-directed specificity are required for high-affinity DNA binding by SATB1. Nucleic Acids Res. 2008;36:2107–2122. doi: 10.1093/nar/gkm1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seto M, Jaeger U, Hockett RD, Graninger W, Bennett S, Goldman P, Korsmeyer SJ. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988;7:123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith MD, Ensor EA, Coffin RS, Boxer LM, Latchman DS. Bcl-2 transcription from the proximal P2 promoter is activated in neuronal cells by the Brn-3a POU family transcription factor. J. Biol. Chem. 1998;273:16715–16722. doi: 10.1074/jbc.273.27.16715. [DOI] [PubMed] [Google Scholar]
- 40.Turner BM. Histone acetylation and an epigenetic code. Bioessays. 2000;22:836–845. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 41.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 42.Gardiner-Garden M, Frommer M. CpG islands in vertebrate genomes. J. Mol. Biol. 1987;196:261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- 43.Wilson BE, Mochon E, Boxer LM. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol. Cell. Biol. 1996;16:5546–5556. doi: 10.1128/mcb.16.10.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang H, Wang J, Boxer LM. Role of the cyclic AMP response element in the bcl-2 promoter in the regulation of endogenous Bcl-2 expression and apoptosis in murine B cells. Mol. Cell. Biol. 2006;26:8599–8606. doi: 10.1128/MCB.01062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mink S, Haenig B, Klempnauer KH. Interaction and functional collaboration of p300 and C/EBPbeta. Mol. Cell. Biol. 1997;17:6609–6617. doi: 10.1128/mcb.17.11.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujii Y, Kumatori A, Nakamura M. SATB1 makes a complex with p300 and represses gp91(phox) promoter activity. Microbiol. Immunol. 2003;47:803–811. doi: 10.1111/j.1348-0421.2003.tb03438.x. [DOI] [PubMed] [Google Scholar]
- 47.Wen J, Huang S, Rogers H, Dickinson LA, Kohwi-Shigematsu T, Noguchi CT. SATB1 family protein expressed during early erythroid differentiation modifies globin gene expression. Blood. 2005;105:3330–3339. doi: 10.1182/blood-2004-08-2988. [DOI] [PubMed] [Google Scholar]
- 48.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 49.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 50.Galande S, Dickinson LA, Mian IS, Sikorska M, Kohwi-Shigematsu T. SATB1 cleavage by caspase 6 disrupts PDZ domain-mediated dimerization, causing detachment from chromatin early in T-cell apoptosis. Mol. Cell. Biol. 2001;21:5591–5604. doi: 10.1128/MCB.21.16.5591-5604.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasui D, Miyano M, Cai S, Varga-Weisz P, Kohwi-Shigematsu T. SATB1 targets chromatin remodelling to regulate genes over long distances. Nature. 2002;419:641–645. doi: 10.1038/nature01084. [DOI] [PubMed] [Google Scholar]
- 52.Gayther SA, Batley SJ, Linger L, Bannister A, Thorpe K, Chin SF, Daigo Y, Russell P, Wilson A, Sowter HM, et al. Mutations truncating the EP300 acetylase in human cancers. Nat. Genet. 2000;24:300–303. doi: 10.1038/73536. [DOI] [PubMed] [Google Scholar]
- 53.Robertson KD. DNA methylation and chromatin - unraveling the tangled web. Oncogene. 2002;21:5361–5379. doi: 10.1038/sj.onc.1205609. [DOI] [PubMed] [Google Scholar]
- 54.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 55.Tan JA, Song J, Chen Y, Durrin LK. Phosphorylation-dependent interaction of SATB1 and PIAS1 directs SUMO-regulated caspase cleavage of SATB1. Mol. Cell. Biol. 30:2823–2836. doi: 10.1128/MCB.01603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.