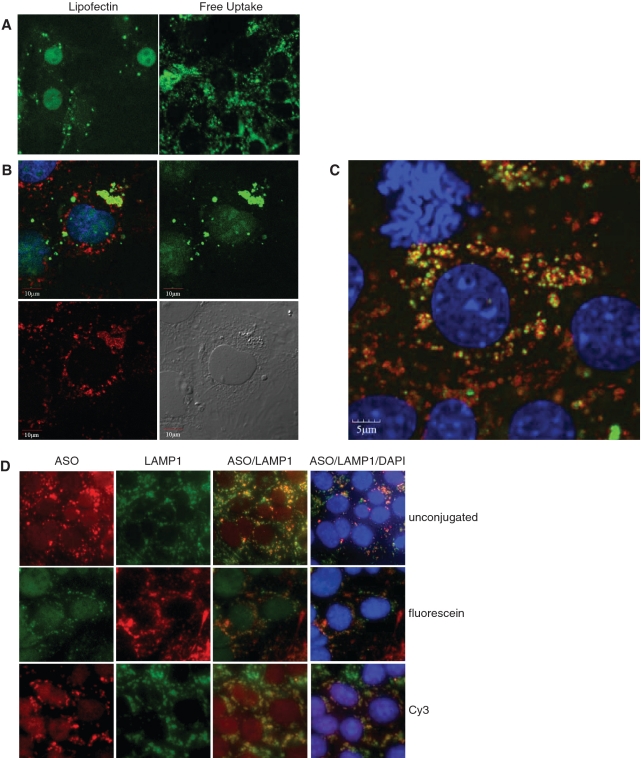
Figure 5.
Localization of fluorescently labeled SSO in MHT cells. (A) Fluorescein labeled SSO was transfected into MHT cells using lipofectin reagent for 4 h followed by 20 h of incubation in the absence of the cationic lipid in complete medium (left panel) or delivered to cells by adding to complete medium for 24 h (right panel). SSO localization is shown in formaldehyde fixed cells. (B) MHT cells were incubated with 100 nM fluorescein labeled SR-B1 SSO (green) for 2 h and cells were fixed and permeabilized. Cells were stained with a LAMP1 antibody (red) to label lysosomal structures and visualized using confocal microscopy. Nuclei of the cells were counterstained with DAPI. (C) MHT cells were incubated with 100 nM fluorescein labeled SR-B1 SSO (green) for 24 h and cells were fixed and permeabilized. Cells were stained with a LAMP1 antibody (red) to label lysosomal structures and visualized using confocal microscopy. Nuclei of the cells were counterstained with DAPI (blue). (D) MHT cells were incubated with 100 nM unlabeled SR-B1 SSO (top row), fluorescein conjugated SR-B1 SSO or Cy3 labeled SR-B1 SSO for 24 h. At the end of the incubation period, cells were fixed and stained for LAMP1 using an unlabeled LAMP1 monoclonal antibody, followed by either a fluorescein (top and bottom rows) or Texas red (middle row) labeled goat anti-mouse antibody. Cells were counterstained with DAPI (blue dye). The unlabeled antibody, was detected using a rabbit polyclonal antibody that recognizes the phosphorothioate backbone present in the SSO followed by a Texas red goat anti-rabbit antibody.