Figure 6.
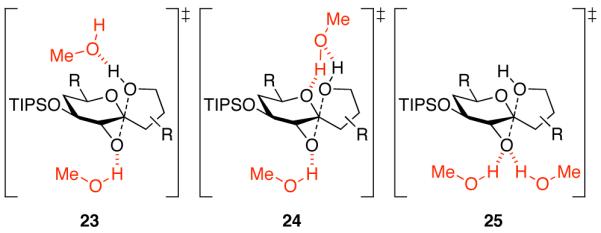
Three possible SN2 transition states for MeOH-catalyzed epoxide-opening spirocyclization with inversion of configuration under MeOH hydrogen-bonding catalysis. In transition state 23, both the epoxide leaving group and alcohol nucleophile are activated with separate MeOH hydrogen bonds. Transition state 24 is similar, but the upper MeOH also engages in a second hydrogen bond to the tetrahydropyran ring oxygen that may disfavor competing SN1 mechanisms involving oxocarbenium formation. In transition state 25, the epoxide electrophile is activated by two MeOH hydrogen bonds, as seen in epoxide hydrolase enzymes.26
