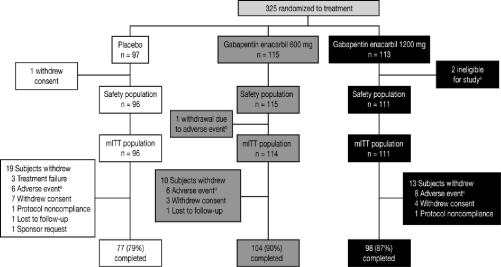
Figure 1.
Subject disposition
a2 subjects were ineligible for study entry due to failed entry criteria at baseline. b1 subject withdrawn due to an adverse event of somnolence. c8 subjects withdrawn due to adverse events of depression (n = 2), hypotension (n = 1), vertigo (n = 1), decreased libido (n = 1), joint sprain (n = 1), sedation (n = 1), and nausea and dizziness (n = 1). d6 subjects withdrawn due to adverse events of somnolence (n = 1), fatigue and somnolence (n = 1), increased platelet count (n = 1), dizziness (n = 1), sedation (n = 1), and hypertension (n = 1). e6 subjects withdrawn due to adverse events of palpitations and chest discomfort (n = 1), mood swings (n = 1), headache (n = 1), pruritis (n = 1), joint swelling (n = 1), and sleep apnea syndrome (n = 1). mITT, modified intent-to-treat.