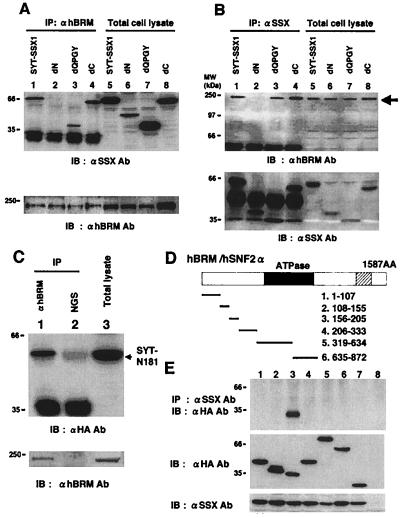
Figure 4.
Analysis of the binding regions of SYT-SSX1 and hBRM/hSNF2α in 293T cells. (A) SYT-SSX1 and its three deletion mutants (Fig. 2) expressed in 293T cells were immunoprecipitated with anti-hBRM/hSNF2α antibody and probed with anti-SSX antibody (Upper, lanes 1–4). Expression levels of SYT-SSX1 and its mutants were examined by immunoblotting (Upper, lanes 5–8). Immunoprecipitants and total cell lysates were also probed with anti-hBRM antibody (Lower). Lysates with 200 μg of proteins were used for immunoprecipitation and 20 μg of total cell lysates were applied for immunoblotting. (B) 293T cell lysates expressing SYT-SSX1 and its mutants were immunoprecipitated with anti-SSX antibody and probed with anti-hBRM antibody (Upper, lanes 1–4) or with anti-SSX antibody (Lower, lanes 1–4). Protein expression levels of endogenous hBRM/hSNF2α (Upper, lanes 5–8) and SYT-SSX1 and its mutants (Lower, lanes 5–8) are displayed. (C) Association of hBRM/hSNF2α with N terminus 181 amino acids of SYT. pcDNA3- HA-GFP-SYT-N181 was transfected to 293T cells and total cell lysates were immunoprecipitated with anti-BRM antibody (lane 1) or normal goat serum (NGS; lane 2) and probed with anti-HA antibody. Expression levels of SYT-N181 are shown in lane 3. Immunoprecipitants and total cell lysates were also probed with anti-hBRM antibody (Lower). (D) Schematic structure of hBRM/hSNF2α and its mutants. The closed frame represents ATPase domain and the shaded frame bromo domain. The number of amino acids comprising six mutants are described. (E) Analysis of the binding region of hBRM/hSNF2α to SYT-SSX1. Six mutants of hBRM were tagged with HA-GFP, coexpressed with SYT-SSX1, and SYT-SSX1 was immunoprecipitated by anti-SSX antibody and probed with anti-HA tag antibody to detect coprecipitated mutants of hBRM/hSNF2α (Top). Confirmations of expression levels of hBRM-derived mutants and SYT-SSX1 are shown in the Middle and Bottom. Lanes 1 to 6 correspond to the number of mutants described above. Lane 7, HA-GFP; lane 8, empty vectors.