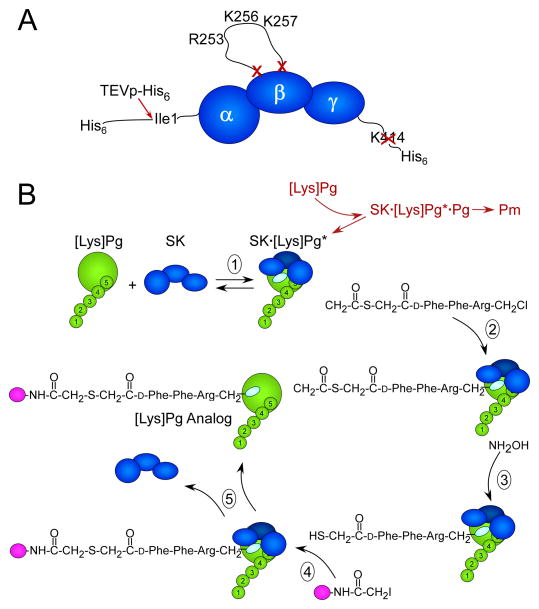
Figure 1. Illustrations of the SK construct used for active site-labeling and the labeling reaction scheme.
A. Modifications of SK to generate SKΔ(R253-L260)ΔK414-His6. SK represented (dark blue) by the α-, β-, and γ-domains from the NH2- to COOH-terminus. The NH2-terminal His6-tag is followed by the TEVp substrate recognition sequence (not shown) immediately adjacent to the essential Ile1- residue of the α-domain that is cleaved by TEVp-His6 (red arrow), removing the His6-segment. Residues Arg253-Leu260 in the SK β-domain containing the kringle 5-binding R253, K256, and K257 residues were deleted (red X, X). The COOH-terminal K414 was also deleted (red X). B. Reactions of the active site-labeling scheme: (1) SK (dark blue) binds to the [Lys]Pg (green) catalytic domain, which is attached to kringles 1–5 (numbered) forming the SK· [Lys]Pg* conformationally activated catalytic site (light blue oval). In coupled reactions (red) that are greatly inhibited by the use of the construct in A, [Lys]Pg binds as a substrate of SK· [Lys]Pg*, forming SK· [Lys]Pg*· [Lys]Pg, which is proteolytically activated to Pm. (2) Nα-[(acetylthio)acetyl]-D-Phe-Phe-Arg-CH2Cl reacts irreversibly with the active site in SK· [Lys]Pg*, trapping and inactivating the complex. (3) Reaction of the inhibitor incorporated into the SK· [Lys]Pg* active site with NH2OH generates a free thiol that in the presence of a fluorescence probe-iodoacetamide (magenta ball) (4) is selectively, covalently modified. (5) The probe-inhibitor-labeled SK· [Lys]Pg* complex is dissociated in NaSCN and the active site-labeled fluorescent [Lys]Pg analog is purified.