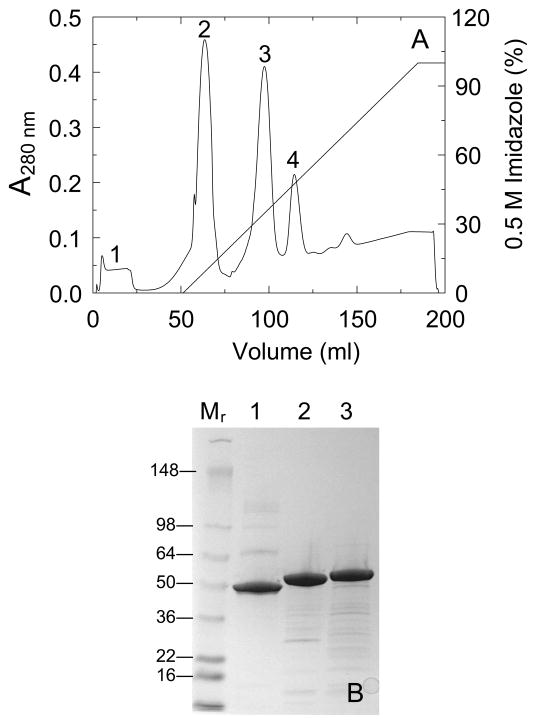
Figure 2. Purification of SKΔ (R253-L260) ΔK414-His6 by Ni2+- iminodiacetic acid-Sepharose chromatography.
A. Elution profile of the products of a reaction of His6-(TEVp cleavage site)-SKΔ (R253-L260) ΔK414-His6 with TEVp-His6 at a 10:1 substrate:protease molar ratio as described in Materials and methods, monitored by the A280 nm-absorbance eluting from a 5 ml Ni2+- iminodiacetic acid-Sepharose FPLC column equilibrated at 22 °C with 50 mM Hepes, 400 mM NaCl, 50 mM imidazole, pH 7.4, followed by a linear gradient of 0.05–0.5 M imidazole in the equilibration buffer. Minor contaminants eluting in the equilibration buffer (peak 1), the TEVp-cleaved protein SKΔ (R253-L260) ΔK414-His6 (peak 2), residual uncleaved His6-(TEVp cleavage site)-SKΔ (R253-L260) ΔK414-His6 (peak 3), and TEVp-His6 (peak 4). B. SDS-PAGE (4–15% gradient gel) of reduced samples (7 μg) of native SK (lane 1), SKΔ (R253-L260) ΔK414-His6 (lane 2), and His6-(TEVp cleavage site)-SKΔ (R253-L260) ΔK414-His6 (lane 3). Molecular mass markers in kDa (lane Mr).