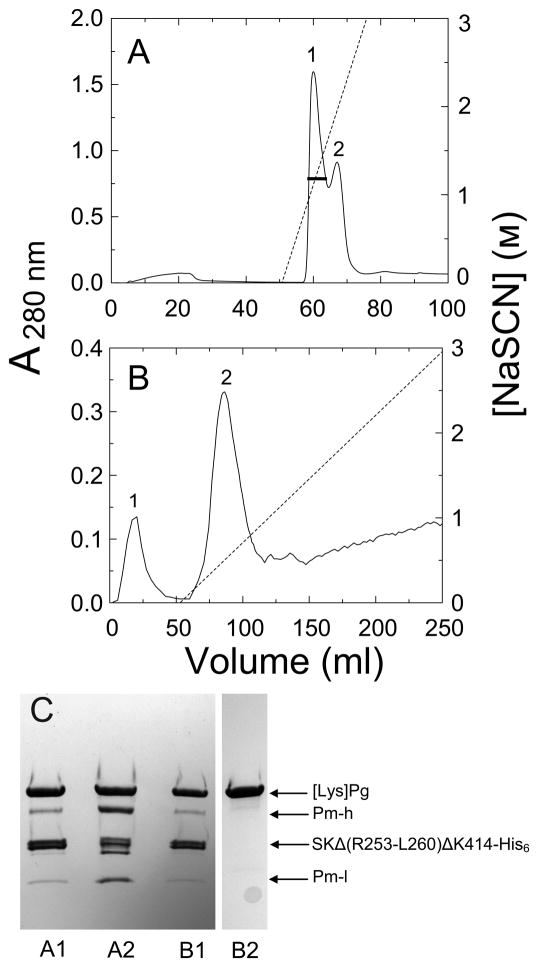
Figure 3. Purification of labeled [Lys]Pg from labeling reaction mixtures by Ni2+-iminodiacetic acid-Sepharose and SK-AffiGel-10 affinity chromatography.
A. Elution profile, measured by A280 nm-absorbance, of [Lys]Pg products from a reaction mixture of 25 μM [Lys]Pg with 50 μM SKΔ (R253-L260) ΔK414-His6 inactivated with 410 μM ATA-FFR-CH2Cl, and subsequently labeled with 155 μM 5-IAF on Ni2+- iminodiacetic acid-Sepharose (5 ml). Proteins were eluted with a steep gradient of NaSCN over 6 column-volumes. The black bar represents the fractions of peak 1 pooled. B. Elution profile of the pooled, concentrated, and dialyzed fractions in A on SK-AffiGel-10 (1 cm 16 cm; 5 mg of SK/ml of gel) as described in Materials and methods. The SK mutant·Pg/Pm complexes elute in the equilibration buffer (peak 1), labeled [Lys]Pg (peak 2) is eluted with a shallow gradient of 3 M NaSCN in the equilibration buffer over 20 column-volumes, and labeled Pm elutes at higher NaSCN (not detectable in this chromatogram). C. SDS-PAGE (4–15% gradient gels) of reduced samples (8–10 μg) from panel A, peak 1 (A1) and peak 2 (A2), and from panel B, peak 1 (B1) and peak 2 (B2). Sample B2 was from the same preparation run on a separate, identical gel. Bands representing labeled [Lys]Pg ([Lys]Pg), SKΔ (R253-L260) ΔK414-His6 (SKΔ (R253-L260) ΔK414-His6), the Pm heavy chain (Pm-h) and labeled light chain (Pm-l) are identified by the arrows.