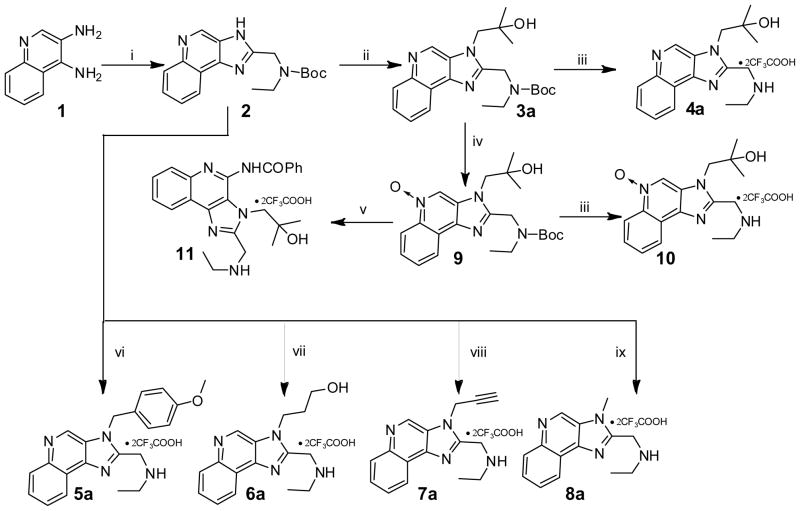
Scheme 1.
Syntheses of derivatives of 4a and N3-substituted 3H imidazoquinolines.
Reagents: i. 2-(tert-Butoxycarbonyl(ethyl)amino)acetic acid, HATU, DMF (b) NaOH/H2O, EtOH; ii. DBU, 2,2-dimethyloxirane; iii. CF3COOH; iv. 3-Chloroperoxybenzoic acid, CH2Cl2, CHCl3, MeOH; v. (a) Benzoyl isocyanate, CH2Cl2 (b) CF3COOH. vi. 1-(Chloromethyl)-4-methoxybenzene, THF, 80 oC (b) CF3COOH; vii. 3-Bromo-1-propanol, DMF, 80 °C (b) CF3COOH; viii. Propargyl bromide, THF, 90 oC (b) CF3COOH; ix. Methyl iodide, DBU, THF (b) CF3COOH.