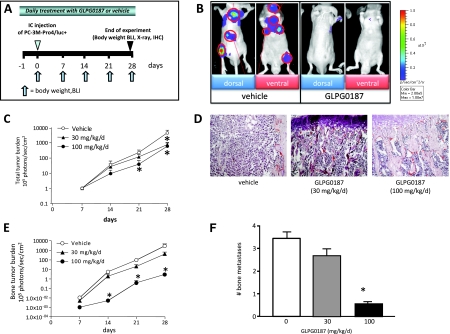
Figure 4.
Effects of systemic administration of GLPG0187 on tumor growth and formation of de novo metastases. (A) Schematic representation of the preventive protocol. Mice were treated daily with either subcutaneously administrated vehicle or GLPG0187 from day -1 onward. At day 0, 100,000 PC-3M-Pro4/luc cells were inoculated into the left heart ventricle, and once a week, body weight was measured and BLI images were taken. (B) Representative images of mice treated with vehicle or 100 mg/kg per day GLPG0187 taken at day 28 after inoculation. (C) Total tumor burden for the mice treated with 30 mg/kg per day GLPG0187 (closed triangles), 100 mg/kg per day GLPG0187 (closed circles), or vehicle (open circles). (D) Dose-dependent reduction in tumor volume in GLPG0187-treated animals as shown by hematoxylin and eosin staining (bone images of vehicle, 30-mg/kg per day, and 100-mg/kg per day GLPG0187-treated animals, respectively). (E) Bone tumor burden for the mice treated with 30-mg/kg per day GLPG0187 (closed triangles), 100-mg/kg per day GLPG0187 (closed circles), or vehicle (open circles). (F) Total number of bone metastases per mouse (n = 10/group; *P < .05).