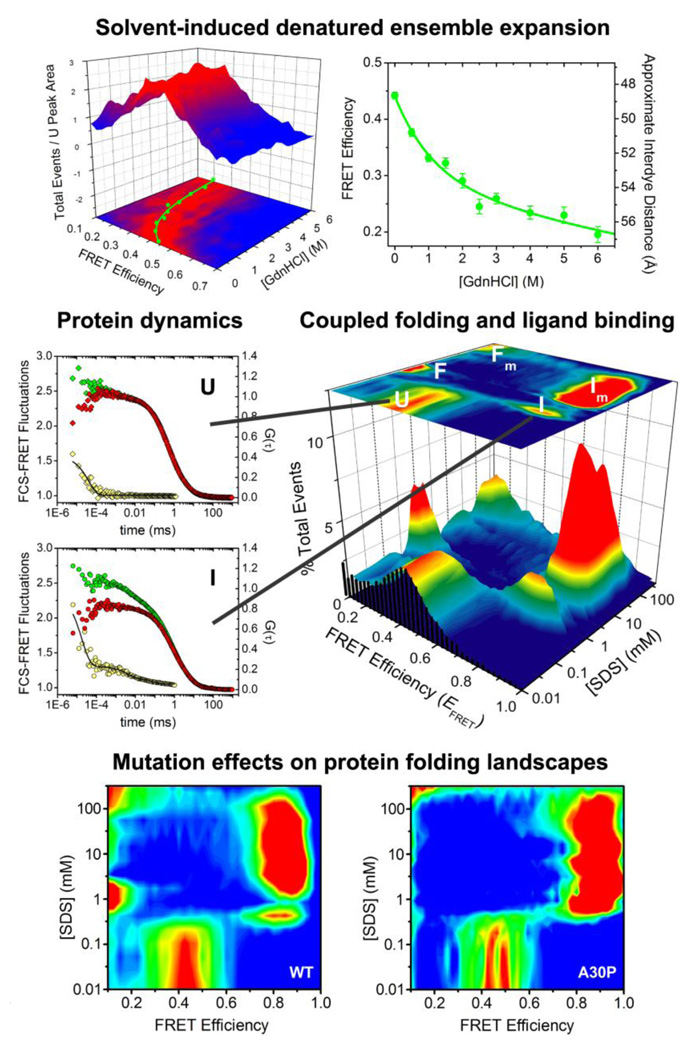
Fig. 3.
Equilibrium, dynamic and binding-folding behavior of an intrinsically disordered protein studied using single-molecule fluorescence. α-Synuclein denaturation demonstrates non-cooperative expansion (top, adapted from [26], Ferreon et al. Methods Enzymol. (2010) 472:179). SDS binding reveals multistate folding and varied dynamics (middle, adapted from [55], Ferreon et al. Proc. Natl. Acad. Sci. USA, (2009) 106:5645). The Parkinson’s disease-linked mutation A30P substantially alters α-synuclein binding-folding landscape (bottom, adapted from [56], Ferreon et al. Angew. Chem. Int. Ed. (2010) 49:3469).