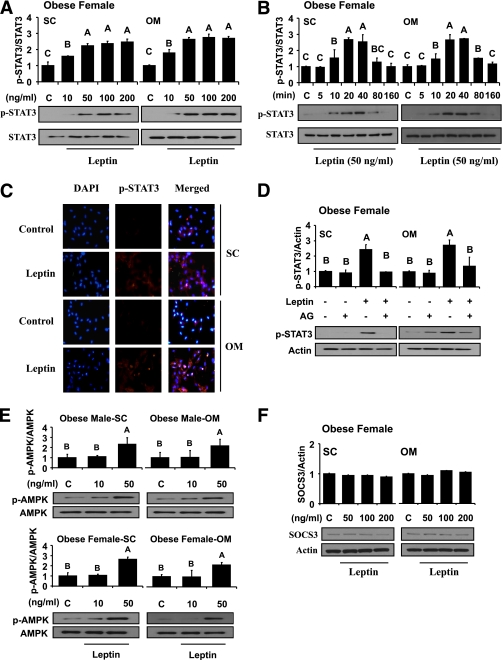
FIG. 3.
Laboratory study III. In vitro metreleptin signaling in subcutaneous (SC) and omental (OM) hPA from lean and obese subjects. In vitro metreleptin administration in hPA was performed as described in detail in research design and methods. A: Cells were treated with metreleptin at the indicated concentrations for 30 min. B: Cells were treated with metreleptin at the indicated times. C: Cells were treated with metreleptin (50 ng/mL) for 30 min. Immunodetection was carried out as described in detail in research design and methods. All pictures were ×40 magnification. D: Cells were pretreated with the STAT3 inhibitor AG490 (AG, 1 μmol/L) for 1 h, followed by treatment with 50 ng/mL metreleptin for 30 min. E and F: Cells were treated with metreleptin at the indicated concentrations for 30 min. All lysates were examined by Western blotting as described in detail in research design and methods. All density values for each protein band of interest are expressed as a fold increase. Data were analyzed using one-way ANOVA followed by post hoc test for multiple comparisons. Values are means (n = 3) ± SD. Means with different letters are significantly different, P < 0.05. (A high-quality digital representation of this figure is available in the online issue.)