Abstract
OBJECTIVE
The pathophysiological mechanisms to explain the association between risk of type 2 diabetes and elevated concentrations of γ-glutamyltransferase (GGT) and alanineaminotransferase (ALT) remain poorly characterized. We explored the association of liver enzymes with peripheral and hepatic insulin resistance, insulin secretion, insulin clearance, and glucagon concentration.
RESEARCH DESIGN AND METHODS
We studied 1,309 nondiabetic individuals from the Relationship between Insulin Sensitivity and Cardiovascular disease (RISC) study; all had a euglycemic-hyperinsulinemic clamp and an oral glucose tolerance test (OGTT) with assessment of insulin secretion and hepatic insulin extraction. The hepatic insulin resistance index was calculated in 393 individuals.
RESULTS
In both men and women, plasma concentrations of GGT and ALT were inversely related with insulin sensitivity (M/I) (all P < 0.01). Likewise, the hepatic insulin resistance index was positively correlated with both GGT (r = 0.37, P < 0.0001, men; r = 0.36, P < 0.0001, women) and ALT (r = 0.25, P = 0.0005, men; r = 0.18, P = 0.01, women). These associations persisted in multivariable models. Increased GGT and ALT were significantly associated with higher insulin secretion rates and with both reduced endogenous clearance of insulin and hepatic insulin extraction during the OGTT (P = 0.0005 in men; P = 0.003 in women). Plasma fasting glucagon levels increased over ALT quartiles (men, quartile 4 vs. quartile 1 11.2 ± 5.1 vs. 9.3 ± 3.8 pmol/L, respectively, P = 0.0002; women, 9.0 ± 4.3 vs. 7.6 ± 3.1, P = 0.001).
CONCLUSIONS
In healthy individuals, increased GGT and ALT were biomarkers of both systemic and hepatic insulin resistance with concomitant increased insulin secretion and decreased hepatic insulin clearance. The novel finding of a positive correlation between ALT and fasting glucagon level concentrations warrants confirmation in type 2 diabetes.
Markers of liver function, specifically γ-glutamyltransferase (GGT) and alanine aminotransferase (ALT), predict incident type 2 diabetes in various populations (1–5). This has been confirmed by a recent meta-analysis that suggested that GGT may be a better diabetes predictor than ALT (6). We recently reported that a moderate elevation of GGT concentration within the normal range is a strong risk marker for incident type 2 diabetes in a large nonobese population, independently of the homeostasis model assessment index (7). However, the physiopathological mechanisms that underlie the association between GGT, ALT, and the risk of diabetes remain poorly understood. Studies have shown that elevated levels of ALT reflect peripheral insulin resistance (8,9), but specific assessment of hepatic insulin sensitivity with appropriate methods is lacking. Furthermore, the relationship between elevated liver markers, including GGT, and both insulin secretion and insulin clearance has not previously been addressed.
The aim of the current study is to determine whether liver markers (GGT and ALT) are mainly associated with peripheral insulin resistance (assessed by the hyperinsulinemic-euglycemic clamp), hepatic insulin resistance (assessed through endogenous glucose production), or insulin secretion (assessed during the oral glucose tolerance test [OGTT]) in a large cohort of healthy men and women participating in the Relationship between Insulin Sensitivity and Cardiovascular disease (RISC) study (10,11). We performed a sex-specific analysis because both GGT and ALT values are classically higher in men compared with women.
RESEARCH DESIGN AND METHODS
RISC is a prospective observational cohort study whose rationale and methodology have been published, as well as the characteristics of the individuals recruited (10,11). Clinically healthy men and women, aged 30–60 years, were recruited from the local population of 19 centers in 14 European countries. Initial exclusion criteria were as follows: treatment for obesity, hypertension, lipid disorders or diabetes, pregnancy, cardiovascular or chronic lung disease, weight change ≥5 kg in the last 6 months, cancer (in last 5 years), and renal failure. Exclusion criteria after screening were as follows: arterial blood pressure ≥140/90 mmHg, fasting plasma glucose ≥7.0 mmol/L, 2-h plasma glucose (following a 75-g OGTT) ≥11.0 mmol/L, total serum cholesterol ≥7.8 mmol/L, serum triglycerides ≥4.6 mmol/L, and electrocardiogram abnormalities. The present analysis is based on the 1,309 participants who satisfied all of the above criteria, whose clamp study passed the quality-control check, and for whom liver markers were available.
Ethics committee approval was obtained by each recruiting center. Volunteers were given detailed written information on the study and an oral explanation, and they all signed a consent form.
Lifestyle and medical history.
Information was collected on smoking and physical activity. Alcohol intake was assessed using a standardized questionnaire and quantified in grams per week. Height, body weight, and BMI were recorded and percent body fat and fat-free mass (FFM) were evaluated by the TANITA bioimpedance balance (Tanita International Division). Obesity was defined as BMI ≥30 kg/m2. Information on physical activity was collected with the 7-day International Physical Activity Questionnaire (IPAQ), a previously validated assessment tool for international studies that provides a comprehensive evaluation of daily physical activity habits (12).
OGTT.
Blood samples were taken before and at 30, 60, 90, and 120 min into the OGTT, together with samples for central analysis of routine blood chemistry. Blood collected during the studies was separated into plasma and serum, aliquoted, and stored at −20°C for glucose and at −80°C for lipids.
Glucose concentrations were measured by the glucose oxidase technique. Plasma insulin and C-peptide were measured by a two-site time-resolved fluoroimmunoassay (AutoDELFIA Insulin kit; Wallac Oy, Turku, Finland) using monoclonal antibodies, with the following assay characteristics (for insulin and C-peptide, respectively): sensitivity 1–2 and 5 pmol/L, within-assay variation 5 and 5%, and between-assay variation 5 and 3.5%. Liver enzymes were centrally assayed on a Dade-Behring Dimension RXL Autoanalyser in Cambridge, U.K. Serum adiponectin was determined in Aarhus, Denmark, by an in-house time-resolved immunofluorometric assay (TR-IFMA) based on two antibodies and recombinant human adiponectin, which measures total circulating adiponectin (including high and low molecular–mass isoforms) (R&D Systems, Abingdon, U.K.) (13). All standards and unknown samples were analyzed in duplicate, with the exception of nonspecific binding, which was analyzed in quadruplicate. The intraassay coefficient of variation (CV) was <5% and the interassay CV was <10%. Glucagon was assayed in Odense, Denmark, by the glucose-oxidase method (Cobas Integra, Roche), which is highly specific for the free C-terminus of the molecule and therefore specific for pancreatic glucagon, with the following assay characteristics: normal range (5–20 pmol/L), sensitivity <1 pmol/L; within-assay CV <5% at 20 pmol/L; and between-assay CV <12% (11).
Insulin clamp.
On a separate day within 1 month of the OGTT, a hyperinsulinemic-euglycemic clamp was performed. Exogenous insulin was administered as a primed-continuous infusion at a rate of 240 pmol/min per m2 simultaneously with a variable 20% dextrose infusion adjusted every 5–10 min to a maintain plasma glucose level within 0.8 mmol/L (±15%) of the target glucose level (4.5–5.5 mmol/L). The clamp procedure was standardized across centers; the data from each clamp study were immediately transferred to the coordinating center where they underwent quality-control scrutiny according to preset criteria.
In this cohort, a subset of 393 individuals had an assessment of fasting endogenous glucose production (EGP) by a continuous infusion of 6,6-[2H]glucose for 2 h in basal before the beginning of the clamp. Plasma samples were collected every 15 min, from 0 to 90 min, and every 5–10 min from 90 to 120 min for the determination of plasma glucose and insulin concentrations, and glucose enrichment was measured by gas chromatography mass spectrometry. Basal EGP was calculated as the ratio between the tracer infusion rate (micromoles per min per kilogram of FFM) and the tracer-to-tracee ratio. The individuals who had an assessment of fasting endogenous glucose production by the tracer infusion were slightly younger (43 ± 8 vs. 44 ± 8 years, P = 0.02) and had a higher BMI and waist circumference (88 ± 14 vs. 86 ± 12 cm, P = 0.01) but did not differ for ALT or GGT activities compared with those who did not have the EGP assessment. The hepatic insulin resistance index was calculated as the product of fasting insulinemia and endogenous glucose production (14,15).
Data analysis.
Insulin sensitivity was expressed as the ratio of the M value during the final 40 min of the 2 h clamp to the mean plasma insulin concentration measured during the same interval (M/I) and normalized to FFM and expressed in units of μmol ⋅ min−1 ⋅ kg FFM−1 ⋅ (nmol ⋅ L−1)−1.
Insulin secretion was calculated during the OGTT by C-peptide deconvolution (16). Total insulin secretion corresponds to the integral of insulin secretion during the entire OGTT.
The endogeneous insulin clearance during the OGTT (in L/min per m2) was defined as follows: (mean insulin secretion) / (mean insulin concentration), where the means were calculated by trapezoids. Hepatic insulin fractional extraction during the OGTT was determined as follows: 1 − (clearance from the clamp) / (endogenous clearance during the OGTT), where insulin clearance from the clamp (in L/min per m2) was calculated as the ratio between insulin infusion and steady-state insulin concentration in the last 40 min of the clamp.
Visit at year 3.
As planned in the design of the cohort, at 3 years each participant was invited for an OGTT; 575 women and 476 men attended this second OGTT following the same methods as described above. Participants who attended this second visit were significantly older than those who did not come back, but there were no differences for waist, fasting glycemia, M/I, GGT, or ALT values at baseline. Impaired fasting glucose was defined as fasting glucose ≥6.1 mmol/L, and impaired glucose tolerance was defined as fasting glucose <7.0 mmol/L and 2-h glucose ≥7.8 mmol/L but <11 mmol/L.
Statistical analysis.
The data are expressed as means ± SD or as median (interquartile range) for variables with a skewed distribution, and categorical data are expressed as percentages. Variables that were not normally distributed were log transformed before analyses. Data were analyzed for men and women separately. The relationships between GGT, ALT, and either insulin sensitivity or insulin secretion were assessed first by linear regression analysis with adjustment for age and recruitment center. Secondly, physical activity, alcohol intake, and waist circumference were added into the regression model. The relations between quartiles of both GGT and ALT activities and each outcome of interest were tested by a multivariable linear regression analysis, adjusting for age, recruitment center, physical activity, alcohol intake, and waist circumference. Logistic regression analysis was used to assess the relationship between liver marker activities and the risk of impaired glucose tolerance at year 3 after adjustment for the same covariates. Statistical analyses used StatView for Windows (version 5.0; SAS Institute, Cary, NC) and SAS (version 9.2; SAS Institute).
RESULTS
Both GGT and ALT values were significantly higher in men than in women (Table 1, P < 0.001 for both). Men had a higher mean BMI than women at baseline.
TABLE 1.
Characteristics of participants: the RISC study
| Men | Women | |
|---|---|---|
| n | 586 | 723 |
| Age (years) | 43 (8) | 44 (8) |
| Current smoker | 162 (28%) | 179 (25%) |
| BMI (kg/m2) | 26.4 (3.5) | 24.8 (4.3) |
| Physical activity (MET-min ⋅ week−1) | 2,212 (960–4,657) | 2,133 (990–4,830) |
| Alcohol intake (g ⋅ week−1) | 74 (30–144) | 30 (11–65) |
| Waist circumference (cm) | 93 (10) | 81 (12) |
| Fasting glucose (mmol ⋅ L−1) | 5.3 (0.8) | 4.9 (0.6) |
| 2-h Glycemia (mmol ⋅ L−1) | 5.7 (1.5) | 5.8 (1.5) |
| Fasting insulin (pmol ⋅ L−1) | 37.0 (20.0) | 33.7 (19.1) |
| Fasting glucagon (pmol ⋅ L−1) | 10 (7–12) | 8 (6–9) |
| Adiponectin (mg ⋅ L−1) | 5.97 (4.62–7.77) | 9.35 (7.17–12.18) |
| GGT (UI ⋅ L−1) | 26 (18–34) | 17 (12–21) |
| ALT (UI ⋅ L−1) | 22 (16–28) | 15 (11–18) |
| M/I (μmol ⋅ min−1 ⋅ kg FFM−1 ⋅ [nmol ⋅ L−1]−1) | 111.4 (80.0–151.0) | 144.6 (107.2–189.6) |
| Hepatic insulin resistance index (pmol ⋅ L−1 ⋅ mmol ⋅ min−1 ⋅ kg FFM−1)* | 0.38 (0.26–0.55) | 0.42 (0.28–0.62) |
| Basal insulin secretion rate (pmol/min per m2) | 74.2 (55.6–97.8) | 66.8 (52.0–86.5) |
| Total insulin secretion rate during OGTT (nmol/m2) | 39.4 (31.5–49.6) | 39.4 (32.2–48.4) |
| Endogenous clearance of insulin during OGTT (L/min per m2) | 1.57 (1.22–1.87) | 1.63 (1.30–2.00) |
| Hepatic insulin extraction during OGTT (dimensionless) | 0.61 (0.51–0.69) | 0.62 (0.54–0.70) |
Data are means (SD) or median (1st–3rd quartile) unless otherwise indicated.
*Calculated in 393 individuals.
Fasting and 2-h glycemia.
In men, fasting glycemia was more strongly correlated with GGT than ALT activity (Table 2). In women, fasting glycemia was more tightly associated with ALT than GGT activity (Table 2). In both sexes, GGT was correlated with 2-h glycemia even after taking into account possible confounding factors: age, center, physical activity, alcohol intake, and waist circumference (P = 0.004 in women and P = 0.037 in men). In contrast, ALT activity was no longer related with 2-h glycemia in either men (P = 0.08) or women (P = 0.10) in multivariable models after further adjustment for the confounding factors cited above.
TABLE 2.
Standardized β-coefficients from linear regression between ALT and GGT activities and metabolic variables: the RISC study
| ALT (IU ⋅ L−1) | GGT (IU ⋅ L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||
| β | P | β | P | β | P | β | P | |
| Fasting glycemia (mmol ⋅ L−1) | 0.08 | 0.046 | 0.22 | <0.0001 | 0.12 | 0.004 | 0.14 | 0.0001 |
| 2-h Glycemia (mmol ⋅ L−1) | 0.13 | 0.001 | 0.13 | 0.0004 | 0.13 | 0.002 | 0.10 | 0.005 |
| Fasting glucagon (pmol ⋅ L−1) | 0.12 | 0.004 | 0.14 | 0.0002 | 0.09 | 0.03 | 0.06 | 0.09 |
| Fasting insulinemia (pmol ⋅ L−1) | 0.33 | <0.0001 | 0.17 | <0.0001 | 0.24 | <0.0001 | 0.16 | <0.0001 |
| M/I (μmol ⋅ min−1 ⋅ kg FFM−1 ⋅ [nmol ⋅ L−1]−1) | −0.18 | <0.0001 | −0.15 | <0.0001 | −0.17 | <0.0001 | −0.11 | <0.0001 |
| Hepatic insulin resistance index (pmol ⋅ L−1 mmol ⋅ min−1 kg FFM−1)* | 0.24 | 0.0003 | 0.16 | 0.029 | 0.28 | <0.0001 | 0.27 | <0.0001 |
| Basal insulin secretion rate (pmol/min per m2) | 0.30 | <0.0001 | 0.18 | <0.0001 | 0.23 | <0.0001 | 0.17 | <0.0001 |
| Total insulin secretion rate during OGTT (nmol/m2) | 0.23 | <0.0001 | 0.12 | 0.001 | 0.18 | <0.0001 | 0.18 | <0.0001 |
| Endogenous clearance of insulin during OGTT (L/min per m2) | −0.14 | 0.0007 | −0.07 | 0.05 | −0.16 | 0.0002 | −0.12 | 0.002 |
| Hepatic insulin extraction during OGTT (dimensionless) | −0.15 | 0.0005 | −0.06 | 0.09 | −0.14 | 0.0005 | −0.09 | 0.02 |
β-Coefficients are adjusted for recruitment center and age.
*Calculated in 393 individuals.
Insulin sensitivity.
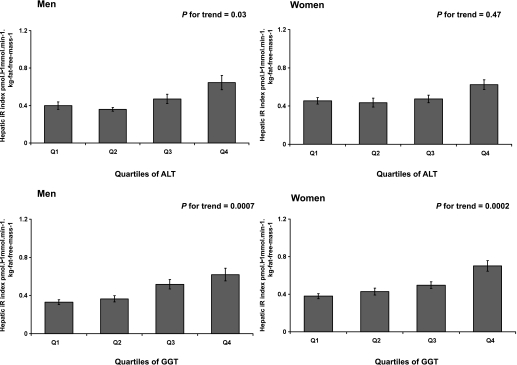
In both men and women, a higher ALT or GGT activity was significantly associated with a lower M/I after adjustment for age and center (Table 2). This association was unchanged in multivariable models. Accordingly, insulin sensitivity decreased across quartiles of both ALT and GGT after adjustment for age, center, physical activity, alcohol intake, and waist circumference (Table 3). Likewise, the hepatic insulin resistance index was positively correlated with both ALT and GGT activity, with a stronger correlation for GGT in both sexes (Table 2, Fig. 1).
TABLE 3.
Metabolic characteristics of men and women during OGTT and euglycemic-hyperinsulinemic clamp according to the quartiles of GGT
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q 1 | Q 2 | Q 3 | Q 4 | P for trend | Q1 | Q2 | Q3 | Q4 | P for trend | |
| Fasting insulinemia (pmol ⋅ L−1) | 23.2 (14.0) | 27.3 (22.0) | 34.0 (25.9) | 34.5 (25.7) | 0.0004 | 23.0 (16.0) | 23.2 (16.0) | 26.2 (19.0) | 31.0 (26.7) | <0.0001 |
| Adiponectin (mg ⋅ L−1) | 6.9 (2.7) | 6.8 (2.4) | 6.2 (2.9) | 5.9 (2.3) | 0.002 | 10.8 (4.1) | 10.2 (3.8) | 9.8 (3.6) | 9.1 (3.7) | 0.001 |
| M/I (μmol ⋅ min−1 ⋅ kg FFM−1 ⋅ [nmol ⋅ L−1]−1) | 130.9 (67.7) | 116.8 (85.1) | 95.5 (65.3) | 93.6 (64.2) | <0.0001 | 145.6 (74.2) | 153.4 (76.7) | 142.6 (89.7) | 134.4 (95.6) | 0.001 |
| Basal insulin secretion rate (pmol/min per m2) | 59.8 (26.7) | 71.1 (34.9) | 82.0 (46.3) | 92.9 (40.5) | 0.002 | 66.5 (29.0) | 68.9 (25.5) | 70.7 (29.7) | 84.9 (34.2) | 0.0008 |
| Total insulin secretion during OGTT (nmol/m2) | 34.0 (14.8) | 37.4 (15.2) | 44.3 (18.3) | 46.1 (18.3) | 0.0029 | 37.7 (12.3) | 41.1 (13.2) | 40.5 (12.8) | 46.1 (15.4) | 0.004 |
| Endogenous clearance of insulin during OGTT (L/min per m2) | 1.73 (0.6) | 1.59 (0.6) | 1.40 (0.7) | 1.44 (0.7) | 0.0005 | 1.67 (0.6) | 1.68 (0.6) | 1.60 (0.7) | 1.47 (0.8) | 0.003 |
| Hepatic insulin extraction during OGTT (dimensionless) | 0.65 (0.1) | 0.62 (0.1) | 0.59 (0.2) | 0.58 (0.2) | 0.004 | 0.63 (0.2) | 0.63 (0.2) | 0.62 (0.1) | 0.60 (0.2) | 0.09 |
Data are (median [interquartile range]) unless otherwise indicated. P values are given after adjustment for age, center, physical activity, alcohol intake, and waist circumference. Quartiles of GGT are as follows: men, 7–20, 21–26, 27–36, and >36 IU ⋅ L−1; and women, 5–13, 14–17, 18–23, and >23 IU ⋅ L−1.
FIG. 1.
Hepatic insulin resistance index for men (left panel) and women (right panel) according to quartiles of both ALT and GGT. Bars show means ± SEM. P value is adjusted for age, center, physical activity, alcohol intake, and waist circumference after a logarithmic transformation.
This association between GGT and both peripheral and hepatic insulin resistance persisted after exclusion of participants with GGT values above the normal range in both sexes. The association between ALT and M/I was no longer significant for men when the analysis was restricted to those with normal values but it persisted for women.
Insulin secretion.
Basal and total insulin secretion rates were significantly associated with both liver markers (Table 2). This association persisted after adjustment for M/I. Insulin secretion increased across quartiles of GGT after adjustment for age, center, physical activity, alcohol intake, waist, and M/I (Table 3). A similar pattern was observed for ALT in men but did not reach statistical significance in women (Table 4). These relationships between liver markers and insulin secretion persisted after exclusion of participants with GGT or ALT values above the normal range in both men and women.
TABLE 4.
Metabolic characteristics of men and women during OGTT and euglycemic-hyperinsulinemic clamp according to the quartiles of ALT
| Men |
Women |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q 1 | Q 2 | Q 3 | Q 4 | P for trend | Q1 | Q2 | Q3 | Q4 | P for trend | |
| Fasting insulinemia (pmol ⋅ L−1) | 24.0 (15.0) | 27.0 (17.0) | 33.0 (23.0) | 41.4 (29.0) | <0.0001 | 24.0 (18.0) | 23.0 (16.0) | 25.0 (19.0) | 30.0 (23.0) | 0.45 |
| Adiponectin (mg/L) | 6.3 (3.4) | 6.4 (3.8) | 6.0 (3.1) | 5.5 (2.6) | 0.14 | 9.4 (4.7) | 10.0 (5.2) | 9.2 (5.0) | 8.8 (5.4) | 0.83 |
| M/I (μmol ⋅ min−1 ⋅ kg FFM−1 ⋅ [nmol ⋅ L−1]−1) | 133.6 (83.3) | 110.8 (64.1) | 109.9 (58.1) | 90.4 (65.3) | 0.003 | 146.4 (98.6) | 160.2 (72.3) | 138.2 (73.7) | 134.7 (75.5) | 0.002 |
| Basal insulin secretion rate (pmol/min per m2) | 61.2 (29.1) | 71.1 (35.0) | 75.6 (45.3) | 94.4 (44.0) | <0.0001 | 60.6 (31.6) | 67.0 (31.1) | 65.6 (33.5) | 76.6 (42.6) | 0.0008 |
| Total insulin secretion during OGTT (nmol/m2) | 35.1 (14.4) | 38.0 (16.1) | 43.0 (18.9) | 45.8 (18.8) | <0.0001 | 38.1 (15.9) | 37.8 (14.7) | 40.9 (15.5) | 41.8 (18.3) | 0.08 |
| Endogenous clearance of insulin during OGTT (L/min per m2) | 1.63 (0.6) | 1.63 (0.7) | 1.57 (0.7) | 1.37 (0.7) | 0.83 | 1.57 (0.7) | 1.74 (0.6) | 1.63 (0.7) | 1.56 (0.8) | 0.67 |
| Hepatic insulin extraction during OGTT (dimensionless) | 0.61 (0.2) | 0.63 (0.1) | 0.63 (0.2) | 0.56 (0.2) | 0.49 | 0.62 (0.2) | 0.63 (0.1) | 0.62 (0.2) | 0.62 (0.2) | 0.17 |
Data are median (interquartile range) unless otherwise indicated. P values are given after adjustment for age, center, physical activity, alcohol intake, and waist circumference. Quartiles of ALT are as follows: men, 3–17, 18–22, 23–29, and >29 IU ⋅ L−1; and women, 2–11, 12–14, 15–19, and >19 IU ⋅ L−1.
Insulin clearance during the OGTT.
Both endogenous clearance of insulin and hepatic insulin extraction during the OGTT were negatively associated with GGT activity after adjustment for age and recruitment center (Table 2). Both decreased across quartiles of GGT, in men and women, in the multivariable model (Table 3). There was no association between insulin clearance and ALT activity in multivariable regression (Table 4).
Glucagon concentration.
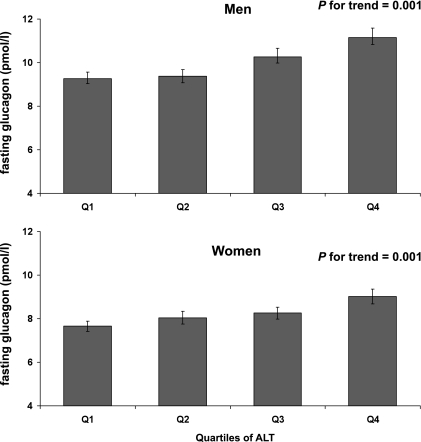
Fasting glucagon level was significantly correlated with ALT concentrations in both men and women after adjustment for age, center, physical activity, alcohol intake, and waist circumference (Table 2, Fig. 2). The relationship between fasting glucagon and GGT levels was weaker, and the association remained significant in the multivariable model in men only (Table 2). In both men and women, the association between fasting glucagon and ALT persisted after adding BMI and waist circumference in the multivariable model (P = 0.01 in men and P = 0.003 in women). This association was independent of GGT activity, M/I, fasting glycemia, and fasting insulinemia.
FIG. 2.
Fasting glucagon concentration for men (top panel) and women (bottom panel) according to quartiles of ALT. Bars show means ± SEM. P value is adjusted for age, center, physical activity, alcohol intake, and waist circumference after a logarithmic transformation.
Impaired glucose tolerance after 3 years.
ALT level was not associated with fasting glycemia at year 3 in either men or women. Baseline GGT was significantly related to fasting glycemia at year 3 in men only (β = 0.12, P = 0.009).
Baseline GGT but not ALT activities were significantly associated with 2-h glycemia at year 3 in both men (β = 0.12, P = 0.01) and women (β = 0.11, P = 0.02) in the multivariable model. Sixty-one men and 78 women had 2-h glucose ≥7.8 mmol/L at year 3. GGT ≥30 UI/L in men and GGT ≥20 UI/L in women were, respectively, associated with a significantly increased risk of impaired glucose tolerance at year 3 (men, odds ratio 2.64 [95% CI 1.42–4.91], P = 0.002; women, 2.03 [1.09–3.78], P = 0.024).
DISCUSSION
The main finding of this study is that GGT and ALT activities were strongly associated with both peripheral and hepatic insulin resistance and reduced hepatic insulin extraction in healthy men and women. A moderate elevation of both GGT and ALT activities within the normal range appears to be a strong marker for insulin resistance, independently of abdominal adiposity or obesity. This is in agreement with a large body of evidence showing a significant association between GGT and ALT activity and incidence of type 2 diabetes in both sexes and in different populations (1–4,7,8).
A previous report in Pima Indians showed that GGT and ALT levels were inversely correlated with peripheral and hepatic insulin sensitivity (17). In the Insulin Resistance Atherosclerosis Study (IRAS) cohort, ALT levels were inversely associated with insulin sensitivity, as assessed by the frequently sampled intravenous glucose tolerance test (9); however, GGT levels were not reported and hepatic insulin resistance was not assessed.
We expand these findings in a European cohort with gold standard methods—the euglycemic-hyperinsulinemic clamp with a tracer infusion—and we show that an elevation in either GGT or ALT is associated with increased systemic and liver insulin resistance in both men and women. Of interest, the mean concentration of GGT in our population was two times lower than that in the obese Pima cohort, extending the relationship between liver enzymes and insulin resistance to normal values. Another report showed that in 75 nondiabetic men, GGT but not ALT activities were inversely related to insulin sensitivity, independently of intra-abdominal fat (18). The sample size was smaller, and insulin sensitivity was assessed by intravenous glucose tolerance test rather than the clamp, which may explain the lack of significant correlation with ALT activity.
The robustness of the association between both GGT and ALT and M/I is substantiated by the fact that the relationship persists in both men and women after exclusion of participants with values above the normal range. A subtle elevation of either liver enzyme could therefore be viewed as an indirect marker of enhanced hepatic insulin resistance and impaired glucose disposal in skeletal muscles. The association between GGT and insulin resistance is not related to the amount of visceral adipose tissue; adjustment for waist circumference did not alter the relationship. However, we cannot formally exclude the absence of substantial fatty liver in the participants because an ultrasonography was not performed. A previous report suggested that the relation between GGT and insulin sensitivity is independent of intra-abdominal fat, as assessed by tomodensitometry (18). Previous studies have shown that diabetic hyperglycemia is related to increased hepatic glucose production and the hepatic insulin resistance index is further increased in the presence of nonalcoholic fatty liver disease (19). In the current study, we have shown a correlation of the hepatic insulin resistance index with ALT and GGT, further explaining the prognostic value of these two variables on the risk of type 2 diabetes.
In our study, the positive association between liver markers and insulin resistance appears to be independent of alcohol intake and persisted after exclusion of heavy drinkers. The decrease in insulin sensitivity observed for those with higher GGT or ALT concentration cannot be related to increased alcohol consumption, given that alcohol intake was positively correlated with M/I in our study (data not shown). Similarly, it has been shown that nondrinkers with high GGT or ALT levels had a higher risk of type 2 diabetes (20). A potential mechanism underlying the association between GGT and insulin resistance may be related to oxidative stress and the role of cellular GGT in the metabolism of extracellular reduced glutathione. It has been proposed that cellular GGT may be involved in the production of reactive oxygen species in the presence of iron or other transition metals (21). Therefore, serum GGT might be considered a sensitive enzyme related to oxidative stress, which is associated with insulin resistance (22).
Fasting glucagon concentration was significantly associated with ALT levels only. Fasting glucagon levels are elevated in type 2 diabetic patients and are related to basal hepatic glucose production and fasting glycemia (14,23).
To our knowledge, the relationship between liver enzymes and glucagon concentration has not previously been investigated. Our findings on a positive correlation between ALT and fasting glucagon levels suggest a putative new mechanism to explain why elevated ALT is predictive of the development of type 2 diabetes (17,24,25). Fasting plasma glucagon level has been shown to be independently associated with whole-body insulin resistance (26). There was a similar trend for GGT to increase across elevated glucagon levels, but the relation appears to be stronger for ALT than for GGT. Plasma ALT is considered to be a more specific marker of hepatocyte alterations than GGT. This positive association of glucagon and ALT appeared to be independent of both fasting glycemia and insulinemia, suggesting a specific yet poorly characterized interaction between the liver and α-cell function. Reciprocally, a possible direct effect of glucagon on ALT secretion in the liver cannot be excluded and deserves further experimental investigations. Experiments in dogs have shown that glucagon chronically impairs hepatic tissue glucose uptake (27). In addition, glucagon increases CYP2E1 mRNA levels in cultured hepatocytes, suggesting a possible pathway by which the glucagon may directly affect the hepatocyte (28). This novel finding of a positive correlation between ALT activity and fasting glucagon concentrations suggests the potential interest of ALT in clinical practice to identify individuals with fasting elevated glucagon secretion, warranting confirmation in type 2 diabetes.
The current study showed that an increase in GGT concentration was associated with diminished hepatic extraction of insulin. This aspect has not previously been recognized. Decreasing rates of hepatic insulin clearance across GGT quartiles may be related to concomitant increased insulin resistance and, consequently, elevated insulin concentrations. Previous studies have underscored an inverse relationship among basal insulin resistance, insulin secretion during a glucose load, and insulin clearance (29–33). During insulin resistance, glucose homeostasis is maintained by an increase in plasma insulin via increased secretion or decreased hepatic insulin extraction. Animal studies indicate that after induction of obesity-mediated insulin resistance, an initial increase in insulin secretion is followed by a decrease in first-pass hepatic insulin extraction (34). The reduction of hepatic insulin extraction with increasing levels of insulin secretion is also explained by a saturation of the insulin receptor on hepatocytes (35). The elevation in GGT activity could be a biological consequence of a chronic high flux of portal venous insulin with increased receptor-mediated insulin endocytosis in the liver.
Besides insulin resistance, we observed a positive and significant correlation between GGT and ALT activities and insulin secretion rates. Increased basal insulin secretion during the OGTT in the participants with higher GGT or ALT levels could be viewed as a direct consequence of enhanced hepatic and systemic hepatic insulin resistance. However, the observation that adjustment for insulin sensitivity did not alter the strong and positive association between liver enzymes and insulin secretion suggests alternative mechanisms such as a possible interaction between the liver and the endocrine pancreas.
Moderate elevations in liver enzymes do not appear to be related to a concomitantly diminished acute insulin response to glucose. Therefore, our findings underscore that the association between liver enzymes and the increased risk of type 2 diabetes observed in a number of epidemiological studies does not directly rely on impaired insulin secretion. However, our cross-sectional data do not preclude the possibility of a subsequent decline in β-cell function over time in those with elevated liver enzymes.
Limitations of the current study include the absence of serial measures of β-cell function over time. Alcohol consumption is difficult to evaluate because of possible underreporting and misclassification. However, the results were not altered after exclusion of heavy drinkers. The strengths of the current study are the large RISC cohort of healthy subjects, the use of the gold standard methodology for measurement of insulin sensitivity with centralized laboratory assays and assessment of insulin secretion, and continuous quality control of data (10). This provided the opportunity to systematically explore, for both men and women, the relationship between liver enzymes and peripheral and hepatic insulin sensitivity, insulin secretion, insulin clearance, and glucagon concentration.
In conclusion, our study shows that in healthy men and women, increased GGT and ALT activities within their physiological ranges are associated with peripheral but also hepatic insulin resistance, increased insulin secretion, and decreased hepatic insulin clearance. Furthermore, ALT activities were positively associated with fasting glucagon concentrations, independently of insulinemia, waist circumference, or insulin sensitivity. These findings confirm the role of the liver in the pathogenesis of type 2 diabetes. The novel finding of a positive correlation between ALT and fasting glucagon concentrations needs to be confirmed in type 2 diabetes.
ACKNOWLEDGMENTS
The RISC study was partly supported by European Union grant QLG1-CT-2001-01252. The European Group for the Study of Insulin Resistance (EGIR) group activities are supported by an unrestricted research grant from Merck Serono, France.
Additional support for the RISC study came from AstraZeneca (Sweden). No other potential conflicts of interest relevant to this study were reported.
F.B. conceived the study, analyzed the results, and wrote the manuscript. P.-H.D. and A.G. revised the manuscript and contributed to discussion. M.L. carried out the study at the center and revised the manuscript. C.H.A., T.K., and A.M. revised the manuscript and contributed to discussion. B.B. contributed to the analysis and discussion and reviewed and edited the manuscript.
APPENDIX
RISC recruiting centers and investigators.
Amsterdam, the Netherlands: R.J. Heine, J. Dekker, S. de Rooij, G. Nijpels, and W. Boorsma; Athens, Greece: A. Mitrakou, S. Tournis, K. Kyriakopoulou, and P. Thomakos; Belgrade, Serbia: N. Lalic, K. Lalic, A. Jotic, L. Lukic, and M. Civcic; Dublin, Ireland: J. Nolan, T.P. Yeow, M. Murphy, C. DeLong, G. Neary, M.P. Colgan, and M. Hatunic; Frankfurt, Germany: T. Konrad, H. Böhles, S. Fuellert, F. Ber, and H. Zuchhold; Geneva, Switzerland: A. Golay, E. Harsch Bobbioni, V. Barthassat, V. Makoundou, T.N.O. Lehmann, and T. Merminod; Glasgow, Scotland: J.R. Petrie (now at Dundee), C .Perry, F. Neary, C. MacDougall, K. Shields, and L. Malcolm; Kuopio, Finland: M. Laakso, U. Salmenniemi, A. Aura, R. Raisanen, U. Ruotsalainen, T. Sistonen, M. Laitinen, and H. Saloranta; London, U.K.: S.W. Coppack, N. McIntosh, J. Ross, L. Pettersson, and P. Khadobaksh; Lyon, France: M. Laville, F. Bonnet (now at Rennes), A. Brac de la Perriere, C. Louche-Pelissier, C. Maitrepierre, J. Peyrat, S. Beltran, and A. Serusclat; Madrid, Spain: R. Gabriel, E.M. Sánchez, R. Carraro, A. Friera, and B. Novella; Malmö, Sweden (1): P. Nilsson, M. Persson, and G. Östling; Malmö, Sweden (2): O. Melander and P. Burri; Milan, Italy: P.M. Piatti, L.D. Monti, E. Setola, E. Galluccio, F. Minicucci, and A. Colleluori; Newcastle-upon-Tyne, U.K.: M. Walker, I.M. Ibrahim, M. Jayapaul, D. Carman, C. Ryan, K. Short, Y. McGrady, and D. Richardson; Odense, Denmark: H. Beck-Nielsen, P. Stehr, K. Hojlund, V .Vestergaard, C. Olsen, and L. Hansen; Perugia, Italy: G.B. Bolli, F. Porcellati, C. Fanelli, P. Lucidi, F. Calcinaro, and A. Saturni; Pisa, Italy: E. Ferrannini, A. Natali, E. Muscelli, S. Pinnola, M. Kozakova, A. Casolaro, and B.D. Astiarraga; Rome, Italy: G. Mingrone, C. Guidone, A. Favuzzi, and P. Di Rocco; Vienna, Austria: C. Anderwald, M. Bischof, M. Promintzer, M. Krebs, M. Mandl, A. Hofer, A. Luger, W. Waldhäusl, and M. Roden.
Project management board.
B. Balkau (Villejuif, France), S.W. Coppack (London, U.K.), J.M. Dekker (Amsterdam, the Netherlands), E. Ferrannini (Pisa, Italy), A. Golay (Geneva, Switzerland), A. Mari (Padova, Italy), A. Natali (Pisa, Italy), J. Petrie (Dundee, Scotland), and M. Walker (Newcastle, U.K.).
Core laboratories and reading centers.
Lipids (Dublin, Ireland): P. Gaffney, J. Nolan, and G. Boran; hormones (Odense, Denmark): C. Olsen, L. Hansen, and H. Beck-Nielsen; albumin:creatinine (Amsterdam, the Netherlands): A. Kok and J. Dekker; genetics (Newcastle-upon-Tyne, U.K.): S. Patel and M. Walker; stable isotope laboratory (Pisa, Italy): A. Gastaldelli and D. Ciociaro; adiponectin, C-reactive protein, and mannose-binding lectin (Odense, Denmark): A. Flyvbjerg; ultrasound reading centre (Pisa, Italy): M. Kozakova; electrocardiogram reading (Villejuif, France): M.T. Guillanneuf; actigraph (Villejuif, France): B. Balkau and L. Mhamdi; data management (Villejuif, France, and Padova and Pisa, Italy): B. Balkau, A. Mari, L. Mhamdi, L. Landucci, S. Hills, and L. Mota; mathematical modeling and Web site management (Padova, Italy): A. Mari, G. Pacini, C. Cavaggion and A. Tura; and the coordinating office (Pisa, Italy): S.A. Hills, L. Landucci, and L. Mota.
Further information on the RISC study and participating centers can be found at www.egir.org.
Footnotes
A complete list of the RISC Study Group can be found in the appendix.
REFERENCES
- 1.André P, Balkau B, Vol S, Charles MA, Eschwège E; DESIR Study Group Gamma-glutamyltransferase activity and development of the metabolic syndrome (International Diabetes Federation Definition) in middle-aged men and women: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) cohort. Diabetes Care 2007;30:2355–2361 [DOI] [PubMed] [Google Scholar]
- 2.Perry IJ, Wannamethee SG, Shaper AG. Prospective study of serum gamma-glutamyltransferase and risk of NIDDM. Diabetes Care 1998;21:732–737 [DOI] [PubMed] [Google Scholar]
- 3.Lee DH, Silventoinen K, Jacobs DR, Jr, Jousilahti P, Tuomileto J. gamma-Glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab 2004;89:5410–5414 [DOI] [PubMed] [Google Scholar]
- 4.Nakanishi N, Suzuki K, Tatara K. Serum gamma-glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care 2004;27:1427–1432 [DOI] [PubMed] [Google Scholar]
- 5.Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Hepatic enzymes, the metabolic syndrome, and the risk of type 2 diabetes in older men. Diabetes Care 2005;28:2913–2918 [DOI] [PubMed] [Google Scholar]
- 6.Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care 2009;32:741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautier A, Balkau B, Lange C, Tichet J, Bonnet F; DESIR Study Group Risk factors for incident type 2 diabetes in individuals with a BMI of <27 kg/m2: the role of gamma-glutamyltransferase. Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetologia 2010;53:247–253 [DOI] [PubMed] [Google Scholar]
- 8.Hanley AJ, Williams K, Festa A, et al. Elevations in markers of liver injury and risk of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes 2004;53:2623–2632 [DOI] [PubMed] [Google Scholar]
- 9.Hanley AJ, Wagenknecht LE, Festa A, D’Agostino RB, Jr, Haffner SM. Alanine aminotransferase and directly measured insulin sensitivity in a multiethnic cohort: the Insulin Resistance Atherosclerosis Study. Diabetes Care 2007;30:1819–1827 [DOI] [PubMed] [Google Scholar]
- 10.Hills SA, Balkau B, Coppack SW, et al. ; EGIR-RISC Study Group The EGIR-RISC Study (The European Group for the study of Insulin Resistance: Relationship between Insulin Sensitivity and Cardiovascular disease risk): I. Methodology and objectives. Diabetologia 2004;47:566–570 [DOI] [PubMed] [Google Scholar]
- 11.Bonnet F, Patel S, Laville M, et al. ; European Group for the study of Insulin Resistance: Relationship between Insulin Sensitivity and Cardiovascular disease risk Study Group Influence of the ACE gene insertion/deletion polymorphism on insulin sensitivity and impaired glucose tolerance in healthy subjects. Diabetes Care 2008;31:789–794 [DOI] [PubMed] [Google Scholar]
- 12.Hallal PC, Victora CG. Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Med Sci Sports Exerc 2004;36:556. [DOI] [PubMed] [Google Scholar]
- 13.Frystyk J, Tarnow L, Hansen TK, Parving HH, Flyvbjerg A. Increased serum adiponectin levels in type 1 diabetic patients with microvascular complications. Diabetologia 2005;48:1911–1918 [DOI] [PubMed] [Google Scholar]
- 14.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 15.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care 2007;30:1544–1548 [DOI] [PubMed] [Google Scholar]
- 16.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 17.Vozarova B, Stefan N, Lindsay RS, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002;51:1889–1895 [DOI] [PubMed] [Google Scholar]
- 18.Wallace TM, Utzschneider KM, Tong J, et al. Relationship of liver enzymes to insulin sensitivity and intra-abdominal fat. Diabetes Care 2007;30:2673–2678 [DOI] [PubMed] [Google Scholar]
- 19.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007;133:496–506 [DOI] [PubMed] [Google Scholar]
- 20.Sato KK, Hayashi T, Nakamura Y, et al. Liver enzymes compared with alcohol consumption in predicting the risk of type 2 diabetes: the Kansai Healthcare Study. Diabetes Care 2008;31:1230–1236 [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Blomhoff R, Jacobs DR., Jr Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res 2004;38:535–539 [DOI] [PubMed] [Google Scholar]
- 22.Park K, Gross M, Lee DH, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care 2009;32:1302–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism 1989;38:387–395 [DOI] [PubMed] [Google Scholar]
- 24.Larsson H, Ahrén B. Glucose intolerance is predicted by low insulin secretion and high glucagon secretion: outcome of a prospective study in postmenopausal Caucasian women. Diabetologia 2000;43:194–202 [DOI] [PubMed] [Google Scholar]
- 25.Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol 1999;277:E283–E290 [DOI] [PubMed] [Google Scholar]
- 26.Ferrannini E, Muscelli E, Natali A, et al. ; Relationship between Insulin Sensitivity and Cardiovascular disease risk (RISC) Project Investigators Association of fasting glucagon and proinsulin concentrations with insulin resistance. Diabetologia 2007;50:2342–2347 [DOI] [PubMed] [Google Scholar]
- 27.Chen SS, Zhang Y, Santomango TS, Williams PE, Lacy DB, McGuinness OP. Glucagon chronically impairs hepatic and muscle glucose disposal. Am J Physiol Endocrinol Metab 2007;292:E928–E935 [DOI] [PubMed] [Google Scholar]
- 28.Woodcroft KJ, Novak RF. The role of phosphatidylinositol 3-kinase, Src kinase, and protein kinase A signaling pathways in insulin and glucagon regulation of CYP2E1 expression. Biochem Biophys Res Commun 1999;266:304–307 [DOI] [PubMed] [Google Scholar]
- 29.Meier JJ, Holst JJ, Schmidt WE, Nauck MA. Reduction of hepatic insulin clearance after oral glucose ingestion is not mediated by glucagon-like peptide 1 or gastric inhibitory polypeptide in humans. Am J Physiol Endocrinol Metab 2007;293:E849–E856 [DOI] [PubMed] [Google Scholar]
- 30.Eaton RP, Allen RC, Schade DS. Hepatic removal of insulin in normal man: dose response to endogenous insulin secretion. J Clin Endocrinol Metab 1983;56:1294–1300 [DOI] [PubMed] [Google Scholar]
- 31.Ferrannini E, Wahren J, Faber OK, Felig P, Binder C, DeFronzo RA. Splanchnic and renal metabolism of insulin in human subjects: a dose-response study. Am J Physiol 1983;244:E517–E527 [DOI] [PubMed] [Google Scholar]
- 32.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 2005;54:1649–1656 [DOI] [PubMed] [Google Scholar]
- 33.Tillil H, Shapiro ET, Miller MA, et al. Dose-dependent effects of oral and intravenous glucose on insulin secretion and clearance in normal humans. Am J Physiol 1988;254:E349–E357 [DOI] [PubMed] [Google Scholar]
- 34.Kim SP, Ellmerer M, Kirkman EL, Bergman RN. Beta-cell “rest” accompanies reduced first-pass hepatic insulin extraction in the insulin-resistant, fat-fed canine model. Am J Physiol Endocrinol Metab 2007;292:E1581–E1589 [DOI] [PubMed] [Google Scholar]
- 35.Knutson VP. Cellular trafficking and processing of the insulin receptor. FASEB J 1991;5:2130–2138 [DOI] [PubMed] [Google Scholar]