Abstract
Conserved pairs of CBS sequence motifs (named after cystathionine β-synthase) found in a wide variety of proteins associate to form Bateman domains. A new study establishes that Bateman domains bind adenosyl compounds and regulate IMP dehydrogenase, CBS, chloride channels, and AMP-activated protein kinase. This discovery reveals how mutations in CBS sequences in these proteins cause hereditary diseases and provides a rich vista of conceptual opportunities for therapies in energy metabolism, obesity, diabetes, cancer, antivirals, and immunosuppression.
What are CBS sequence motifs?
Alexander Bateman first recognized cystathionine β-synthase (CBS) sequences by looking for internal sequence duplications within proteins of the Methanococcus janaschii genome. These CBS sequences, typically 60 residues in length, occur as tandem pairs in a diversity of proteins, from archaebacteria to eukaryotes (1, 2), including IMP dehydrogenase (IMPDH), whose protein crystal structure shows that CBS sequence pairs form a discrete structural domain (3) termed a Bateman domain (Figure 1) (4). Although it is known that mutations in CBS sequences alter the control functions of their parent proteins and cause hereditary diseases (5–7), the precise mechanisms involved were unknown. Mutations in CBS cause homocystinuria; IMPDH mutations cause retinitis pigmentosa; mutations in chloride channels (CLC1, CLC2, CLC5, and CLCKB) cause a variety of conditions, including congenital myotonia, idiopathic generalized epilepsy, hypercalciuric nephrolithiasis, and classic Bartter syndrome; mutations in the AMP-activated protein kinase (AMPK) γ2 subunit cause cardiac conductance problems similar to Wolff-Parkinson-White syndrome; and mutations in Hampshire pig γ3 cause skeletal muscle glycogen storage disease.
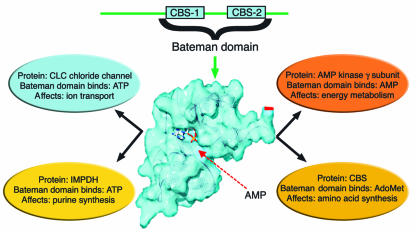
Figure 1.
Pairs of CBS sequences within a protein fold into Bateman domains that bind adenosyl compounds and that mediate the allosteric control of their parent proteins. Shown is the surface-modeled Bateman domain formed by the CBS-1 and CBS-2 sequences of the AMPK γ1 subunit with AMP present in the central binding pocket (4). AdoMet, S-adenosyl methionine.
Bateman domains bind adenosyl compounds
In this issue of the JCI, Scott and colleagues (8) have now found the missing link by showing that pairs of CBS sequences derived from AMPK, IMPDH-2, the chloride channel CLC2, and CBS bind adenosyl compounds (Figure 1). They show that AMPK, with its two pairs of CBS sequences, binds two molecules of AMP or ATP; the single pair of CBS sequences present in IMPDH allows it to bind a single mole of ATP, as does the pair of CBS sequences in CLC2. CBS binds a single mole of S-adenosyl methionine. The authors introduced known hereditary mutations into CBS sequences of the parent protein and found that they caused dramatic shifts in ligand-binding properties, which explained the loss of allosteric control of the parent protein. These findings will also influence research into the wider family of CBS-containing proteins (2) that includes other ion transporters, hemolysin, and poly(A) polymerase.
Regulation of IMPDH by ATP
IMPDH catalyzes the first committed step in the purine nucleoside-synthesis pathway for the generation of GMP. It converts IMP at the active site to xanthosine monophosphate, which is subsequently converted to GMP by GMP synthase. IMPDH activity is increased in proliferating cell types including cancers and activated peripheral blood lymphocytes. Because of this, IMPDH has been an important target for the design of anticancer and immunosuppressive drugs aimed at preventing cancer cell or lymphocyte proliferation. Drugs that inhibit IMPDH (e.g., ribavirin, myco-phenolic acid, and VX-497) reduce cellular GTP and deoxy-GTP levels and can also have broad antiviral activities against both RNA viruses, such as respiratory syncytial virus, and DNA viruses, such as hepatitis B virus and human cytomegalovirus (9). Scott et al. (8) show that IMPDH is activated by ATP binding to its pair of CBS sequences (evidenced by a fourfold increase in Vmax) and propose that this dependence on ATP acts as a checkpoint to ensure that guanine nucleotide synthesis only proceeds when sufficient ATP is available. Much of the IMPDH inhibitor design has focused on the NAD+ cofactor– and IMP substrate–binding pockets within the active site. The findings of Scott et al. (8) now provide additional options for the design of IMPDH inhibitors that target the ATP-binding (allosteric activator) site; these options may offer improved specificity for immunosuppressive, anticancer, and antiviral chemotherapy. IMP also serves as a precursor for AMP synthesis via adenylsuccinate. In this pathway, AMP inhibits conversion of IMP to adenylsuccinate. This begs the question of whether adenylsuccinate could also bind the IMPDH ATP-binding site and activate IMPDH. If so, this would provide a further tier of control between the GMP and AMP synthetic pathways.
Regulating energy metabolism via AMPK
The metabolic stress-sensing protein kinase AMPK has come to prominence recently for its role in the control of metabolism and gene transcription in response to exercise. It is responsible for regulating multiple metabolic pathways, including glucose transport, glycolysis, fatty acid, cholesterol, and triglyceride synthesis, as well as fatty acid oxidation. In addition, AMPK is activated by the adipocyte-derived hormones adiponectin and leptin together with two important drugs used to treat type 2 diabetes, metformin and rosiglitazone (10, 11). For these reasons there has recently been intense interest in AMPK as a target for the treatment of obesity, type 2 diabetes, and the metabolic syndrome.
AMPK consists of three subunits, a catalytic subunit α, an intracellular targeting subunit β, and a regulatory subunit γ. As mentioned above, the γ subunits contain four copies of the CBS sequences. The γ subunits are encoded by three genes (γ1, γ2, and γ3), and mutation in the γ3 gene CBS sequence-1 causes the common Hampshire pig glycogen storage disease previously termed the RN– (Rendement Napole) mutation (12). Economically important alleles within the CBS-1 sequence of the γ3 gene have now been found, which confer reduced muscle glycogen and enhanced meat quality (13). While no corresponding human γ3 mutations have been reported, six families have been identified with mutations in AMPK γ2 (expressed in the heart) that give rise to a cardiac phenotype with Wolff-Parkinson-White syndrome features (14). Scott et al. (8) have demonstrated that the γ subunit, with its two pairs of CBS sequences, binds two molecules of AMP and that the human mutations in these CBS sequences cause loss of AMP binding. Several earlier studies provided hints that the γ subunits were important for regulation by AMP but did not prove it. Hamilton et al. (15) introduced the equivalent γ3 mutation found in Hampshire pigs into γ1 and rendered the AMPK constitutively active, independent of AMP. Cheung et al. (16) showed that AMP could be chemically cross-linked to the γ subunit. While mutations in AMPK γ2 cause a reduction in AMP binding, Scott et al. (8) found that they do not result in constitutively active AMPK, so it is not yet clear how mutations in AMPK γ2 cause the cardiac phenotype.
The crystal structure of IMPDH has allowed 3D modeling of the γ subunit CBS protein sequences by Scott et al. (8) and Adams et al. (4). A putative AMP-binding pocket comprising Arg-70, His-151, Arg-152, and Arg-171 has been identified in the γ1 subunit CBS-1/CBS-1 sequence pairs (4). The presence of multiple basic residues in the binding site is also seen in the classical phosphorylase AMP site (17), the first example of an allosterically regulated enzyme, reported by Cori and Green 60 years ago (18). Of the important basic residues identified in the γ1 subunit AMP-binding site, Arg-70 corresponds precisely to the site of one of the human γ2 mutations, as well as the Hampshire pig γ3 mutation and the original CBS mutation, D444N (5).
Knowledge of how AMPK binds AMP paves the way for rational drug design of AMPK activators and inhibitors
The binding of AMP to the γ subunit of AMPK influences the activation of AMPK in several ways. Activation of AMPK depends on phosphorylation by the tumor-suppressor protein kinase LKB1, which is mutated in most cancer patients with Peutz-Jeghers syndrome (19, 20). The binding of AMP makes AMPK a better substrate for LKB1, in addition to making it more resistant to inactivation by phosphatases. The likely link between AMPK and LKB1 in tumor suppression and cell-growth arrest adds yet another dimension to the potential applications of therapeutic drugs based on the γ subunit AMP-binding site. With regard to health promotion, AMPK mediates a number of the benefits of exercise for metabolism and gene transcription, and this suggests that AMPK-activating drugs that target the γ subunit may increase exercise capacity.
The mark of an important discovery is that it changes our way of thinking. There are now well over a thousand known proteins that contain Bateman domains. For many of these proteins we can now begin to hypothesize about how they are regulated by adenosyl metabolites.
Acknowledgments
Bruce Kemp is supported by a Federation Fellowship from the Australian Research Council and by grants from the National Health and Medical Research Council and the National Heart Foundation, and sponsored by the Max Planck Research Award program.
Footnotes
See the related article beginning on page 274.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: cystathionine β-synthase (CBS); IMP dehydrogenase (IMPDH); chloride channel (CLC); AMP-activated protein kinase (AMPK).
References
- 1.The CBS domain web page. The Sanger Institute. http://www.sanger.ac.uk/Users/agb/CBS/CBS.html.
- 2.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem. Sci. 1997;22:12–13. doi: 10.1016/s0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- 3.Zhang R, et al. Characteristics and crystal structure of bacterial inosine-5′-monophosphate dehydrogenase. Biochemistry. 1999;38:4691–4700. doi: 10.1021/bi982858v. [DOI] [PubMed] [Google Scholar]
- 4.Adams, J., et al. 2004. Intrasteric control of AMPK via the gamma-1 subunit AMP allosteric regulatory site. Protein Sci. In press. [DOI] [PMC free article] [PubMed]
- 5.Kluijtmans LA, et al. Defective cystathionine beta-synthase regulation by S-adenosylmethionine in a partially pyridoxine responsive homocystinuria patient. J. Clin. Invest. 1996;98:285–289. doi: 10.1172/JCI118791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pusch M. Myotonia caused by mutations in the muscle chloride channel gene CLCN1. Hum. Mutat. 2002;19:423–434. doi: 10.1002/humu.10063. [DOI] [PubMed] [Google Scholar]
- 7.Bowne SJ, et al. Mutations in the inosine monophosphate dehydrogenase 1 gene (IMPDH1) cause the RP10 form of autosomal dominant retinitis pigmentosa. Hum. Mol. Genet. 2002;11:559–568. doi: 10.1093/hmg/11.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott JW, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Invest. 2004;113:274–284. doi:10.1172/JCI200419874. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markland W, McQuaid TJ, Jain J, Kwong AD. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob. Agents Chemother. 2000;44:859–866. doi: 10.1128/aac.44.4.859-866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 11.Kemp BE, et al. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 12.Milan D, et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science. 2000;288:1248–1251. doi: 10.1126/science.288.5469.1248. [DOI] [PubMed] [Google Scholar]
- 13.Ciobanu D, et al. Evidence for new alleles in the protein kinase adenosine monophosphate-activated gamma(3)-subunit gene associated with low glycogen content in pig skeletal muscle and improved meat quality. Genetics. 2001;159:1151–1162. doi: 10.1093/genetics/159.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gollob MH, Green MS, Tang AS, Roberts R. PRKAG2 cardiac syndrome: familial ventricular preexcitation, conduction system disease, and cardiac hypertrophy. Curr. Opin. Cardiol. 2002;17:229–234. doi: 10.1097/00001573-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton SR, et al. An activating mutation in the gamma1 subunit of the AMP-activated protein kinase. FEBS Lett. 2001;500:163–168. doi: 10.1016/s0014-5793(01)02602-3. [DOI] [PubMed] [Google Scholar]
- 16.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem. J. 2000;346:659–669. [PMC free article] [PubMed] [Google Scholar]
- 17.Sprang SR, Withers SG, Goldsmith EJ, Fletterick RJ, Madsen NB. Structural basis for the activation of glycogen phosphorylase b by adenosine monophosphate. Science. 1991;254:1367–1371. doi: 10.1126/science.1962195. [DOI] [PubMed] [Google Scholar]
- 18.Cori GT, Green AA. Crystalline muscle phosphorylase II prosthetic group. J. Biol. Chem. 1943;151:31–38. [Google Scholar]
- 19.Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawley SA, et al. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. PMID: 14511394. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]