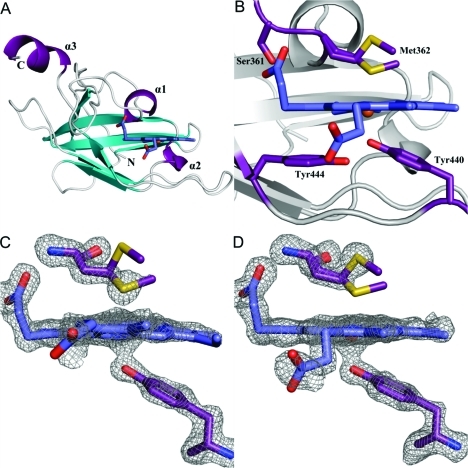
Figure 2.
Crystal structure of the heme-bound C-terminal NEAT domain of IsdB. (A) Overall structure of holo-IsdB-N2 (chain A) viewed down the heme binding pocket. The backbone is shown as a cartoon with helices colored purple, β-strands cyan, and loops gray. Heme is shown protruding from the pocket as sticks, with carbon atoms colored dark blue and oxygen atoms red. One conformation of heme is shown for the sake of clarity. The N- and C-termini and helices are labeled. (B) Close-up of the heme pocket. Residues directly involved in binding the heme molecule (Tyr440, Met362, and Ser361) or indirectly involved in binding (Tyr444) are shown as sticks and labeled. Protein carbon, oxygen, and sulfur atoms are colored purple, red, and yellow, respectively. Heme carbon, oxygen, nitrogen, and iron atoms are colored blue, red, dark blue, and dark red, respectively. (C and D) Fo – Fc omit maps (contoured at 3σ) for Tyr440, Met362, and heme of chains A and B.