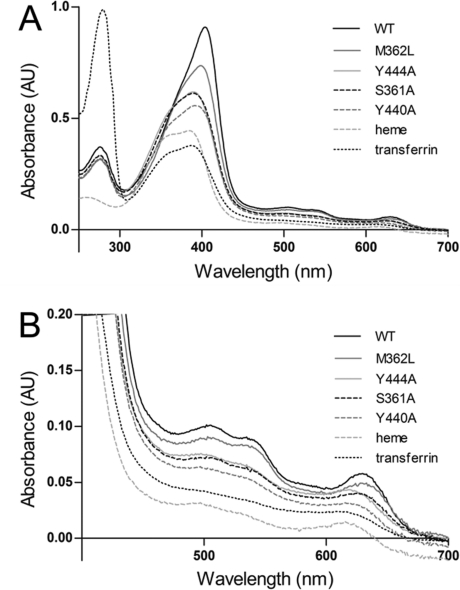
Figure 3.
Visible spectra of 5 μM holo-IsdB-N2 WT and variants. Heme (5 μM) was added to apoprotein (5 μM) and allowed to reach equilibrium at room temperature. Spectra of heme (5 μM) alone in buffer and heme (5 μM) added to transferrin, a non-heme binding protein, are also shown for comparison. (A) Spectra of all proteins recorded between 250 and 700 nm. The absorbance at 280 nm is similar for all IsdB-N2 proteins (∼14 kDa), whereas that of transferrin (∼80 kDa) is significantly higher; heme absorbs much less at 280 nm. The spectrum in the Soret range (∼400 nm) is noticeably different for most variants, except S361A and Y444A, which are largely indistinguishable. (B) Close-up of the spectra between 400 and 700 nm revealing characteristic markers of the heme-iron environment. Spectra of WT and M362L are most similar to each other, whereas the spectra of Y440A, Y444A, and S361A resemble the spectrum of free heme.