FIGURE 7.
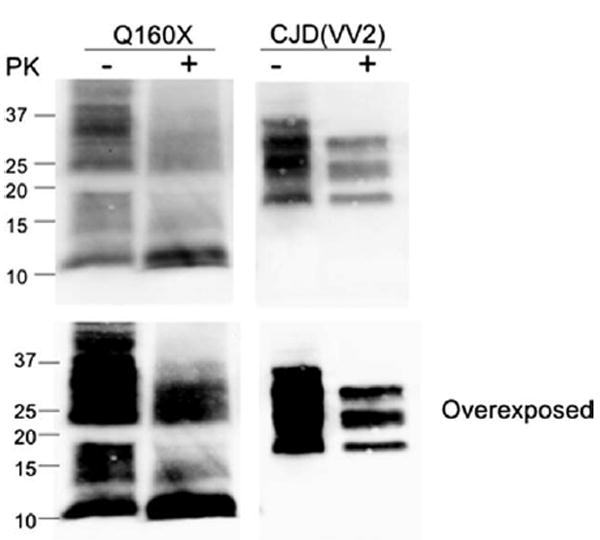
Western blot from proband brain tissue. Western blot analysis of enriched fractions of prion protein scrapie isoform (PrPSc) from brain samples of the PRNP-Q160X patient and a sporadic Creutzfeldt-Jakob disease (sCJD) subject with the 129VV genotype and type 2 proteinase K (PK)-resistant PrPSc (unglycosylated fragment ~19kDa) is shown. This enrichment reveals 2 major fractions of PrP that correspond to residual nonmutated (25–35kDa) PrP and full-length and cleaved (smear between 11 and 18kDa) mutated PrP. The predicted size of mature PrP-Q160X is ~17–18kDa, represented by the top of the second major fraction, with smaller fragments likely representing endogenously cleaved products of the PrP-Q160X mutated protein, the most prominent of which is ~11kDa. None of these smaller fractions is present in sCJD, even with overexposure, suggesting that they result directly from mutated PrP. Following PK treatment (+) the ~11kDa fragment remains most prominent. The smear of PK-resistant PrP that ranges from ~21 to 35kDa may represent PrP-Q160X oligomers or nonmutated PrP converted to PK-resistant fragments by PrP-Q160X. Blots were probed with antihuman PrP monoclonal antibody 3F4.
