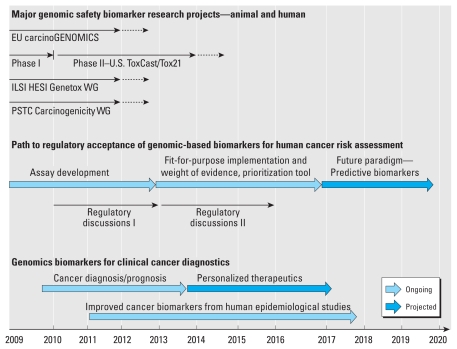
Figure 1.
Road map for human cancer risk assessment. Four major projects that aim to develop a genomic biomarker approach for human cancer risk assessment have been initiated in the United States and the European Union (EU). carcinoGENOMICS (EU’s Sixth Framework Programme, funded from 2009 to 2012) attempts to develop human cell-based in vitro models and tools to detect carcinogens. The Phase I and the Phase II studies, conducted by the U.S. EPA (ToxCast program) and the National Institutes of Health (Tox21 program), exploit available biochemical and molecular assays to develop high-throughput, predictive assay panels. The International Life Sciences Institute Health and Environmental Sciences Institute (ILSI HESI) Genotoxicity Working Group (Genotox WG) focuses on developing an in vitro genomic biomarkers that can provide mechanistic context to in vitro genotoxicity testing. The aim of the FDA’s Critical Path Initiative Predictive Safety Testing Consortium Carcinogenicity Working Group (PSTC Carcinogenicity WG) is to develop an in vivo genomic biomarker detecting hepatocarcinogenicity, applicable for use in early drug development. These initiatives are expected to yield biomarkers and assays that initially could be applied as weight of evidence in a fit-for-purpose manner. This will provide the time and opportunity for refining the methods and assembling a new testing paradigm. Concurrently, progress in the development of cancer diagnostic methods is expected to provide data specific to human cancer that, after integration with in vitro and preclinical genomics data, will open additional avenues to further increase human relevance of this new testing paradigm. Solid lines indicate current funding activities; dotted lines indicate anticipated continuation of funding or projects into the future.