Abstract
The in vitro tissue culture and micropropagation studies for Morus spp., a pivotal sericulture plant, are well established. The rapid and reproducible in vitro response to plant growth regulator treatments has emerged as an essential complement of transformation studies for this plant species. A major area of study is the use of protoplast culture and fusion techniques where advantages to mulberry improvement can be applied. The advancements in genetic transformation of mulberry are reviewed, and a section on strategy for transforming plastids (chloroplasts) of mulberry is included. A role for mulberry in “molecular farming” is envisioned. The conclusions and future prospects for improvement of this economically important tree species are proposed.
Key words: molecular farming, Morus spp., plastid transformation, protoplast electrofusion, sericulture
The importance of silk production is well recognized in sericulture industry that involves cultivation of host plants for silkworm rearing. India is one of the countries where sericulture is an important agro-based cottage industry involved in production of five different silk types—mori, muga, eri, tasar and oak types. This classification comes from type of host plant that act as feed for silkworm, and thus sericulture industry largely depends on the availability of host plant species. Bombyx mori (mulberry silkworm) feeds on mulberry leaves, Philosomia ricini (eri silkworm) on castor leaves, Anthraea assama (muga silkworm) on som and soalu leaves, Anthraea proylei (temperate/oak tasar silkworm) on oak leaves, and Anthraea mylitta (tropical tasar silkworm) on Terminalia leaves. A systematic and proper cultivation of novel primary and/or secondary host plants showing high yield, suitability to silkworm rearing, and resistance to different abiotic stress conditions i.e., tolerance to water stress, alkalinity and salinity are recommended for sericulture improvement.
The genus Morus (commonly known as mulberry) belongs to the family Moraceae, is a group of dioecious woody trees/shrubs. Many varieties of these species are cultivated on a commercial scale in India, China, Japan and Korea for the sericulture industry.1 In India, six species are found, namely, M. alba L., M. indica L., M. nigra L., M. atropurpurea Roxb., M. serrata Roxb. and M. laevigata Wall.2 Due to higher economic return and greater employment potential, attempts are been made to increase productivity by developing high yielding mulberry varieties. At present, Mysore local, Bomaypiasbari, Kanva-2 (K2), Bilidevalaya, Kajli, Sujanpur-1 (S1), BC (2) 59, C776, RFS-175, S36 and Victory-1 are being cultivated extensively in different parts of India.
Studies on Protoplast to Plant Regeneration in Mulberry
The experiments were performed to improve protoplast yield from mesophyll cells of three mulberry genotypes, K2, S13 and S36.3 We have identified important parameters that contribute to the maximum protoplast yield in these mulberry genotypes. The protoplast yield in mulberry was genotype-dependent with 12–13 h of enzyme incubation found to be favorable for the genotype S36 whereas, 9 h of incubation was sufficient for the release of maximum number of intact protoplasts from the genotypes, K2 and S13.3 The concentration of osmoticum mannitol was 0.5 M in the enzyme mixture containing cellulase (2%) and macerozyme (1%). These enzyme concentrations and the level of osmoticum were found to be optimal for the isolation of protoplasts from all mulberry genotypes that were studied. A protocol has also been described for rapid isolation of protoplasts (4 h) from callus cultures of mulberry.4
A protocol for regenerating plants from mulberry protoplasts was established for the genotype S36.5 The first cell divisions were observed at day 4 with cell division frequency (CDF) ranging from 1 to 29% as on day 6. With the density of approximately 1.0 × 105 protoplasts, the combination of zeatin and 2,4-D induced highest percentage of cell divisions (29%) followed with zeatin and NAA (10%). Although the mesophyll protoplasts underwent high initial CDF, their sustained divisions were arrested. The protoplast cultures grown in media supplemented with 13.5 µM dicamba were capable of further cell divisions and colony formation. We have shown a more specific role of the auxin dicamba in inducing cell divisions in mulberry that is different from other auxins like NAA and 2,4-D.5 The protoplast derived colonies formed microcalli which further proliferated into larger calli on the medium containing TDZ and IAA. After third subculture on this medium, up to 2 shoots were regenerated. These shoots were rooted on MS medium with 4.9 µM IBA. A low survival rate of the regenerated shoots was observed under the greenhouse conditions.5
Studies on Protoplast Fusion in Mulberry
The success in plant somatic hybridization depends on availability of suitable technique for protoplast fusion. Different methods have been tried to fuse plant protoplasts. Of these, only polyethylene glycol (PEG) has received widespread acceptance as a fusogen of plant protoplasts. The electric field can be used to fuse protoplasts and moreover it is superior to PEG-induced protoplast fusion in the following aspects: simplicity, less toxicity, less physical damage to the protoplasts, large fusion volume and fine control of the fusion process.6
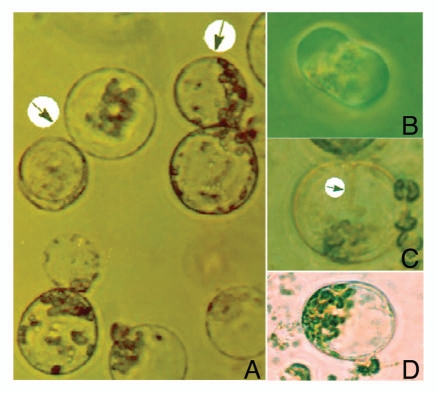
The work on protoplast fusion in mulberry was demonstrated by using chemical fusogens like the PEG and high pH-Ca2+.7 We have tested the possibility of using electrofusion technique for protoplast fusion in mulberry. For this, protoplasts isolated from axenic shoot cultures of two mulberry genotypes, S13 and S36 were fused under electric field conditions. An electric AC field of 50 V/cm (15 sec duration) was necessary for proper alignment of the protoplasts (Fig. 1A). The closer membrane contact resulted from application of DC, 400–450 V/cm (70 µs duration) (Fig. 1B). The post-fusion AC field (10 V/cm) was maintained for the duration of 5 sec. The fused protoplasts (hybrids/heterokaryons) regenerated a cell wall following 2–3 days of culture in dark.
Figure 1.
(A) Protoplast alignment following application of AC electric field and (B) DC electric field results into closer membrane contact of the fused protoplast pair, resulting in appearance (C) of distinct cytoplasmic connection (arrow) and fused protoplasts (D).
We have tested the possibility to fuse protoplasts of different sizes that could facilitate monitoring the fusion events. The protoplasts of small size (genotype S36) were fused with larger size protoplasts (genotype S13). A 1:1 mixture (1 to 2 × 105/ml) of mesophyll protoplasts isolated from genotypes S36 and S13 was pipetted into electrofusion chamber under sterile conditions. The electrode system had a 3-mm distance between parallel plate electrodes, mounted on a glass microscope slide and this was placed in 9 cm Petri dish. Protoplasts were allowed to settle and left undisturbed for 10 min in the chamber. The electric fields used for fusion were generated by BTX Electro Cell Manipulator (ECM) 2001, Electro cell fusion and Electroporation System; BTX, Inc., San Diego, CA. Individual heterokaryons were identified by microscopic observation for the appearance of distinct cytoplasmic connection formed between protoplasts in contact, or from the phenomenon of cell sphering8,9 (Fig. 1C and D).
Studies on Genetic Transformation in Mulberry
The genetic engineering by DNA-mediated gene transfer requires efficient transformation systems for the introduction of useful genes into specific mulberry cultivars. Only few attempts were made to transform mulberry plants. Among these were studies on mulberry plants transformed with Agrobacterium tumefaciens, A. rhizogenes, particle bombardment mediated gene delivery and electroporation.
Plastid Transformation in Mulberry: Future Perspectives
Plant genetic engineering via the nucleus is a mature technology that has emerged as a major approach for cultivar improvement as well as for studying gene functions in plants. In contrast, the ability to engineer chloroplast as an alternative site for the expression of foreign genes, proteins, reactions and products has gained prominence relatively recently. Genetic transformation of chloroplast is advantageous because proteins in chloroplast accumulate to high levels, multiple genes may be expressed as polycistronic units, and lack of pollen transmission in most crops results in transgene containment.10
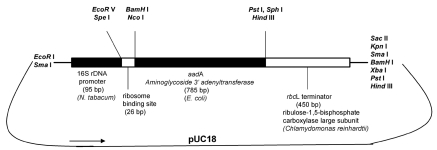
Chloroplast transformation is accomplished through a multistep process. The transformation vector used contains selective plastid markers, spectinomycin-streptomycin and kanamycin resistance, conferred by the expression of chimeric aadA11 (Fig. 2) and aphA6,12 genes, respectively. The gene has to be equipped with appropriate expression signals in the new genetic background, in the case of plastid transformation (a) with an appropriate plastid promoter and (b) a transcription terminator. The construct has then to be equipped with flanking sequences (each 600–1,000 bp) at 5′ and 3′ ends, respectively. These flanking sequences are derived from chloroplast DNA from the place in the chromosome where insertion of the selection cassette is desired. The flanking sequences allow for homologous recombination (gene disruption or replacement) in chloroplast. The drugs used in the selection inhibit chlorophyll accumulation and shoot formation on plant regeneration medium. The transformed lines can be identified by their ability to form green shoots on bleached wild-type leaf sections.10,13
Figure 2.
The design of aminoglycoside-3′-adenyltransferase (aadA) construct for plastid transformation containing 16S rDNA promoter (from Nicotiana tabacum) and rbcL terminator signal (from Chlamydomonas reinhardtii) cloned into pUC18 vector backbone. Restriction sites are indicated. The aadA gene confers spectinomycin and/or streptomycin resistance to the transformed cells.
One of the most exciting potential applications of chloroplast transformation in mulberry will be the production of recombinant proteins for industrial, pharmaceutical or other value-added uses. In addition to mulberry being an important sericulture crop, it is also becoming clear that it could play a major role in “molecular farming”. The following agronomical features make it an excellent target to aid studies of molecular farming:
-
(a)
High yield of leaf biomass and multiple harvests
-
(b)
Sustainable production of the aimed biomolecules for long time without replanting
-
(c)
Compared to annual crops, relatively high resistance to environmental conditions and pest attacks
-
(d)
Low input cost for growth maintenance
Conclusions and Future Directions for Mulberry Improvement
The biotechnology has now provided tools which allow us to select and engineer superior mulberry varieties with much the same speed and efficiency as can be applied to other organisms. Great efforts were made to study different in vitro cultivation methods for mulberry. The several of these protocols already served as useful experimental systems for cell culture and transformation studies in mulberry. Although used for tissue culture and nuclear transformation studies, the use of mulberry for chloroplast transformation has not been reported. Consequently, the next few years are likely to see large and rapid changes in moribiotechnology.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12035
References
- 1.Wakhlu AK, Singhbhau B. A review of tissue culture studies in mulberry (Morus) Sericologia. 2000;40:1–20. [Google Scholar]
- 2.Bhattacharya E, Ranade SA. Molecular distinction amongst varieties of mulberry using RAPD and DAMD profiles. BMC Plant Biol. 2001;1:3. doi: 10.1186/1471-2229-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umate P, Rao AV, Yashodhara V, Rama Swamy N, Sadanandam A. Evaluation of specific parameters in the isolation of protoplasts from mesophyll cells of three mulberry cultivars. Sericologia. 2000;40:469–474. [Google Scholar]
- 4.Umate P, Rao AV, Yashodhara V, Rama Swamy N, Sadanandam A. A simple protocol for rapid and efficient isolation of protoplast from callus cultures of mulberry (Morus indica L.) cv. S13. Sericologia. 2000;40:647–651. [Google Scholar]
- 5.Umate P, Venugopal Rao K, Kiranmayee K, Jaya Sree T, Sadanandam A. Plant regeneration of mulberry (Morus indica) from mesophyll-derived protoplasts. Plant Cell Tiss Org Cult. 2005;82:289–293. [Google Scholar]
- 6.Cheng J, Saunders JA. Methods in Molecular Biology. Plant Cell Electroporation and Electrofusion Protocols. 1995;55:181–186. doi: 10.1385/0-89603-328-7:181. [DOI] [PubMed] [Google Scholar]
- 7.Chand PK, Sahoo Y, Patnaik SK, Patnaik SN. Evaluation of procedures for interspecific protoplast fusion in mulberry. Indian J Seric. 1996;35:46–49. [Google Scholar]
- 8.Sowers AE. Membrane electrofusion: a paradigm for study of membrane fusion mechanisms. Methods Enzymol. 1993;220:196–211. doi: 10.1016/0076-6879(93)20083-f. [DOI] [PubMed] [Google Scholar]
- 9.Reichert NA, Liu D. Protoplast isolation, culture and fusion protocols for kenaf (Hibiscus cannabinus) Plant Cell Tiss Org Cult. 1996;44:201–210. [Google Scholar]
- 10.Khan MS, Maliga P. Fluorescent antibiotic resistance marker for tracking plastid transformation in higher plants. Nat Biotechnol. 1999;17:910–915. doi: 10.1038/12907. [DOI] [PubMed] [Google Scholar]
- 11.Svab Z, Maliga P. High frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA. 1993;90:913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang FC, Klaus SM, Herz S, Zou Z, Koop HU, Golds TJ. Efficient plastid transformation in tobacco using the aphA-6 gene and kanamycin selection. Mol Genet Genom. 2002;268:19–27. doi: 10.1007/s00438-002-0738-6. [DOI] [PubMed] [Google Scholar]
- 13.Umate P, Schwenkart S, Karbat I, Dal Bosco C, Mlcochova L, Volz S, et al. Deletion of PsbM in tobacco alters the QB site properties and the electron flow within photosystem II. J Biol Chem. 2007;282:9758–9767. doi: 10.1074/jbc.M608117200. [DOI] [PubMed] [Google Scholar]