Abstract
Transition to the flowering stage is precisely controlled by a few classes of regulatory molecules. BROTHER OF FT AND TFL1 (BFT) is a member of FLOWERING LOCUS T (FT)/TERMINAL FLOWER 1 (TFL1) family, an important class of flower development regulators with unidentified biochemical function. BFT has a TFL1-like activity and plays a role in axillary inflorescence development. To elucidate the expression pattern of BFT, we analyzed the subcellular localization and conditional expression of BFT in this study. We generated 35S::BFT:GFP plants to investigate the subcellular localization of BFT protein. 35S::BFT:GFP plants showed late flowering, similarly as did 35S::BFT plants. BFT:GFP fusion protein was localized in the nucleus and the plasma membrane, which was different from the localization pattern of FT and TFL1. BFT expression was induced by abiotic stress conditions. ABA, drought, and osmotic stress treatments induced BFT expression, whereas cold, salt, and heat stress conditions did not, suggesting that BFT plays a role in regulating flowering time and inflorescence structure under drought conditions. The induction pattern of BFT was different from those of other FT/TFL1 family genes. Our studies indicated that BFT showed a distinct expression pattern from its homologous genes during the vegetative growth in Arabidopsis.
Key words: flowering time, flowering locus T, terminal flower 1, brother of FT and TFL1, abiotic stress, subcellullar localization
The FLOWERING LOCUS T (FT)/TERMINAL FLOWER 1 (TFL1) family is a small gene family whose members play a pivotal role in flower development in Arabidopsis. The family includes FT, TFL1, TWIN SISTER OF FT (TSF), Arabidopsis thaliana CENTRORADIALIS homologue (ATC), MOTHER OF FT AND TFL1 (MFT) and BROTHER OF FT AND TFL1 (BFT).3,5,6,9,15,17 FT is a floral promoter that integrates signal inputs from various pathways that regulate flowering time in Arabidopsis.5,6 TFL1 plays an antagonistic role to that of FT, functioning as a floral inhibitor. Unlike FT, TFL1 also plays an important role in controlling plant architecture by regulating the expression of LEAFY (LFY) and APETALA1 (AP1), two important floral meristem identity genes in the shoot apical meristem (SAM).3,7 TSF regulates flowering by a mechanism similar to that of FT, although a lesion in TSF does not have an apparent effect on the determination of flowering time. MFT has a weak FT-like activity.17 ATC acts as a floral repressor, and its role is similar to that of TFL1.9 Finally, BFT has a TFL1-like activity, in spite of its amino acid homology to FT,2,4,16 and functions redundantly with TFL1 in inflorescence meristem development in Arabidopsis.16 Although genetic studies elucidated an intricate role of the FT/TFL1 family genes, not much is known about the expression pattern of the remaining members except FT and TFL1. Here, we report that BFT expression showed a distinct pattern from its homologous genes during the vegetative phase. BFT:GFP fusion protein was detected in the nucleus and the plasma membrane. BFT expression was induced by abiotic stress conditions, distinct from other FT/TFL1 family genes, raising the possibility that BFT plays a role in regulating flowering time and inflorescence structure under drought conditions.
BFT is Localized in the Nucleus and the Plasma Membrane
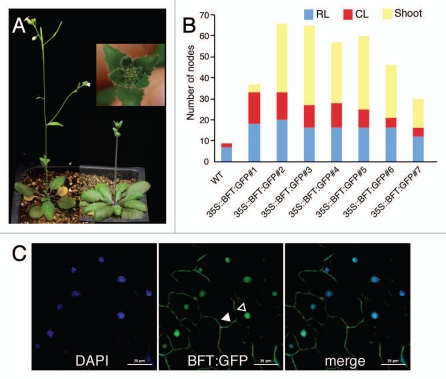
To investigate the subcellular localization of BFT, we expressed a BFT:GFP chimeric gene under the control of the 35S promoter in wild-type Columbia plants. 35S::BFT:GFP transgenic plants showed late flowering (24.4 ± 7.1 leaves) under long-day (LD) conditions (cf. wild-type plants = 10.0 ± 0.8 leaves) (Fig. 1A), similarly as did 35::BFT plants.16 In addition, 35S::BFT:GFP plants produced compact inflorescences in the primary inflorescence filled with leaf primordia surrounded by serrated leaves with trichomes, which is very similar to that produced in TFL1-overexpressing plants.13 35S::BFT:GFP plants also showed a delayed conversion from shoot to flower, thus producing more nodes (Fig. 1B). This suggested that the phase transition in both the vegetative phase and the adult phase was delayed. These observation suggested that functional BFT protein was produced in 35S::BFT:GFP plants. When we analyzed GFP signal under confocal laser microscopy, we identified BFT:GFP signal localized in the nucleus and the plasma membrane (Fig. 1C). This expression pattern was different from those of FT:GFP and HA:TFL1 reported previously. FT:GFP fusion protein was localized in the nucleus and the cytoplasm,1 whereas HA:TFL1 fusion protein was localized in the plasma membrane, vacuole, and dense vesicles approximately 100 nm in diameter.11 Subcellular localization of the remaining FT/TFL1 family members is unknown. The nuclear and plasma membrane localization of BFT:GFP indicated that BFT expression pattern was distinct but had some of the features of both FT and TFL1. Considering that FT directly binds to FD, a bZIP transcription factor,1,14 to activate the floral identity gene AP1, it is tempting to speculate that BFT may compete with FT to bind to FD within the nucleus.
Figure 1.
Phenotype of 35S::BFT:GFP plants and the subcellular localization of BFT:GFP fusion protein. (A) Morphology of a 35S::BFT:GFP transgenic plant (right) and a wild-type Columbia plant (left). Inset showed a close-up view of the primary inflorescence of 35S::BFT:GFP plants. Note that overall phenotype of 35S::BFT:GFP plants is very similar to that of a strong line of 35S::BFT plants.16 (B) Number of nodes formed in the primary shoot of 35S::BFT:GFP transgenic plants. Results from different lines in the T1 generation are shown. CL, cauline leaf; RL, rosette leaf; WT, wild-type Columbia plants. (C) The subcellular localization of BFT in the leaf of 12-day-old 35S::BFT:GFP plants. Note that BFT:GFP fusion protein is detected in the nucleus (open arrowhead) and the plasma membrane (closed arrowhead). Scale bar: 20 µm.
Induction of BFT Expression Under Abiotic Stress Conditions
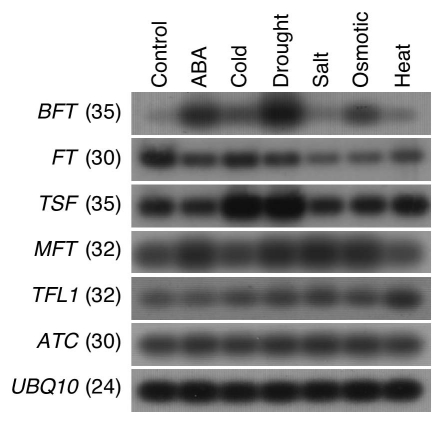
Since the BFT expression level was low in wild-type plants,16 we tested the possibility that BFT expression was conditional. Among the abiotic stress conditions that we tested, BFT expression was upregulated by the ABA stress, drought, and osmotic stress treatments (Fig. 2), whereas the cold, salt stress, and heat stress conditions did not induce BFT expression. This result demonstrated that BFT expression was significantly enhanced by water-related stress. We also monitored the expression levels of other FT/TFL1 family genes. The ATC and TFL1 expression levels were not significantly altered under the above-mentioned stress conditions. FT expression was not induced by the conditions under which BFT expression was enhanced. On the contrary, FT expression was weakly downregulated under salt, osmotic, and heat stress conditions. A notable result was that TSF expression was markedly induced under cold and drought conditions. Collectively, it was apparent that none of the FT/TFL1 family genes showed induction patterns similar to that of BFT, suggesting that BFT also plays a role in regulating flowering time and inflorescence structure under drought conditions.
Figure 2.
Induction of BFT expression revealed by semi-quantitative RT-PCR. For this experiment, wild-type Columbia plants were planted on half-strength Murashige and Skoog (MS) media and grown for 8 days under LD conditions. For abscisic acid (ABA) stress treatment, wild-type seedlings were placed in 100 µM ABA solution for 3 h. For cold treatment, the plants were placed under white light for 40 h in a cold room maintained at 4°C. Drought treatment was provided in a drying station as described previously.10 The seedlings were placed in 100 mM NaCl solution and 100 mM mannitol for 3 h for salt stress and osmotic stress treatments, respectively. For heat stress treatment, the seedlings were placed under white light for 1 h in a growth chamber maintained at 37°C. Expression patterns of the other FT/TFL1 family genes were also monitored. Numbers in parenthesis indicate the number of RT-PCR cycles. UBQ10 was used as an internal control.
It has been suggested that there is a close positive correlation between flowering time and water-use physiology.8 The degree of plasticity of flowering time is suggested to be an important component of fitness.12 Thus, BFT may be suggested to be one of the genes that pleiotropically affect drought physiology and are responsible for differences in flowering time. We are currently conducting experiments to test whether drought tolerance is closely associated with alteration in BFT activity and whether BFT is involved in abiotic stress signaling.
Acknowledgements
This work was supported by Creative Research Initiatives (R16-2008-106-01001-0) of MEST/KOSEF (J.H.A.) and by the Crop Functional Genomics Center (CG1211) of MEST/KOSEF (J.S.L.). K.S.C., S.J.Y. and S.Y.Y. were supported by the BK21 program.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12415
References
- 1.Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- 2.Ahn JH, Miller D, Winter VJ, Banfield MJ, Lee JH, Yoo SY, et al. A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J. 2006;25:605–614. doi: 10.1038/sj.emboj.7600950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. Inflorescence commitment and architecture in Arabidopsis. Science. 1997;275:80–83. doi: 10.1126/science.275.5296.80. [DOI] [PubMed] [Google Scholar]
- 4.Hanzawa Y, Money T, Bradley D. A single amino acid converts a repressor to an activator of flowering. Proc Natl Acad Sci USA. 2005;102:7748–7753. doi: 10.1073/pnas.0500932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, et al. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- 7.Liljegren SJ, Gustafson-Brown C, Pinyopich A, Ditta GS, Yanofsky MF. Interactions among APETALA1, LEAFY and TERMINAL FLOWER1 specify meristem fate. Plant Cell. 1999;11:1007–1018. doi: 10.1105/tpc.11.6.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKay JK, Richards JH, Mitchell-Olds T. Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Mol Ecol. 2003;12:1137–1151. doi: 10.1046/j.1365-294x.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- 9.Mimida N, Goto K, Kobayashi Y, Araki T, Ahn JH, Weigel D, et al. Functional divergence of the TFL1-like gene family in Arabidopsis revealed by characterization of a novel homologue. Genes Cells. 2001;6:327–336. doi: 10.1046/j.1365-2443.2001.00425.x. [DOI] [PubMed] [Google Scholar]
- 10.Nambara E, Kawaide H, Kamiya Y, Naito S. Characterization of an Arabidopsis thaliana mutant that has a defect in ABA accumulation: ABA-dependent and ABA-independent accumulation of free amino acids during dehydration. Plant Cell Physiol. 1998;39:853–858. doi: 10.1093/oxfordjournals.pcp.a029444. [DOI] [PubMed] [Google Scholar]
- 11.Sohn EJ, Rojas-Pierce M, Pan S, Carter C, Serrano-Mislata A, Madueno F, et al. The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proc Natl Acad Sci USA. 2007;104:18801–18806. doi: 10.1073/pnas.0708236104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stinchcombe JR, Dorn LA, Schmitt J. Flowering time plasticity in Arabidopsis thaliana: a reanalysis of Westerman & Lawrence (1970) J Evol Biol. 2004;17:197–207. doi: 10.1046/j.1420-9101.2003.00641.x. [DOI] [PubMed] [Google Scholar]
- 13.Sung ZR, Chen L, Moon YH, Lertpiriyapong K. Mechanisms of floral repression in Arabidopsis. Curr Opin Plant Biol. 2003;6:29–35. doi: 10.1016/s1369-5266(02)00014-6. [DOI] [PubMed] [Google Scholar]
- 14.Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, et al. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol. 2005;46:1175–1189. doi: 10.1093/pcp/pci151. [DOI] [PubMed] [Google Scholar]
- 16.Yoo SJ, Chung KS, Jung SH, Yoo SY, Lee JS, Ahn JH. BROTHER OF FT AND TFL1 (BFT) has a TERMINAL FLOWER 1 (TFL1)-like activity and functions redundantly with TFL1 in inflorescence meristem development in Arabidopsis. Plant J. 2010 doi: 10.1111/j.1365-313X.2010.04234.x. In press. [DOI] [PubMed] [Google Scholar]
- 17.Yoo SY, Kardailsky I, Lee JS, Weigel D, Ahn JH. Acceleration of flowering by overexpression of MFT (MOTHER OF FT AND TFL1) Mol Cell. 2004;17:95–101. [PubMed] [Google Scholar]