Abstract
Many prey modify traits in response to predation risk and this modification of traits can influence the prey's resource acquisition rate. A predator thus can have a “nonlethal” impact on prey that can lead to indirect effects on other community members. Such indirect interactions are termed trait-mediated indirect interactions because they arise from a predator's influence on prey traits, rather than prey density. Because such nonlethal predator effects are immediate, can influence the entire prey population, and can occur over the entire prey lifetime, we argue that nonlethal predator effects are likely to contribute strongly to the net indirect effects of predators (i.e., nonlethal effects may be comparable in magnitude to those resulting from killing prey). This prediction was supported by an experiment in which the indirect effects of a larval dragonfly (Anax sp.) predator on large bullfrog tadpoles (Rana catesbeiana), through nonlethal effects on competing small bullfrog tadpoles, were large relative to indirect effects caused by density reduction of the small tadpoles (the lethal effect). Treatments in which lethal and nonlethal effects of Anax were manipulated independently indicated that this result was robust for a large range of different combinations of lethal and nonlethal effects. Because many, if not most, prey modify traits in response to predators, our results suggest that the magnitude of interaction coefficients between two species may often be dynamically related to changes in other community members, and that many indirect effects previously attributed to the lethal effects of predators may instead be due to shifts in traits of surviving prey.
It has long been recognized that the effects of a predator can extend further than the species they consume. For example, killing a prey can indirectly affect other predators of the prey (causing exploitative competition) and resources of the prey (causing trophic cascades and keystone predator effects). Such indirect effects can have a large influence on the structure and dynamics of ecological communities (refs. 1–5 and reviewed in refs. 6–9). Traditionally, ecologists have ascribed these indirect effects of predators almost solely to their effects on prey density.
Predators, however, also interact with prey by inducing changes in prey phenotype. Prey often respond to changes in predator density by modifying traits as diverse as behavior (10–12), morphology (12), and life history (12). These trait-changes may, in turn, affect other species interacting with these prey (13–18). For example, a predator-induced reduction in prey activity (making it a less efficient forager) may indirectly affect prey resources and consequently prey competitors. To distinguish such “nonlethal” predator effects from density-mediated indirect interactions (DMIIs, ref. 17) caused by chains of effects on density (“lethal” effects) alone, these types of indirect interactions have been termed trait-mediated indirect interactions (TMIIs, ref. 17).
Evidence is accumulating that TMIIs can have significant effects on the dynamics of ecological communities. For example, studies performed in both aquatic and terrestrial systems have shown that nonlethal affects of a predator on prey foraging rates can indirectly affect resources, competitors, and other predators of the prey (see, e.g., refs. 18 and 19 and references therein). Typically such TMIIs have been demonstrated by presenting only a signal (cue) of the presence of a predator, or examining assemblages of species at life history stages where lethal effects are minimal, to isolate the trait effects. These studies have provided important insights into the mechanisms underlying TMIIs and their potential importance.
A critical issue that largely has gone unexplored, however, is how nonlethal effects contribute to the net effect of a predator (but see refs. 20–22), i.e., what is the relative contribution of the TMIIs to the net indirect effects of the predator? The total impact of a prey on resources (and therefore on competitors of the prey) is a function of prey density and the average foraging rate of each individual prey. The predator affects both of these factors, and these effects are then transmitted to the resources and competitors. One might argue that nonlethal effects are important, if at all, only when they are relatively large and lethal effects are small; i.e., lethal effects could overwhelm nonlethal effects even though the latter appear large when lethal effects are negligible. However, there are straightforward reasons to believe that nonlethal effects of the predator can be large: The foraging reduction due to the presence of the predator (the nonlethal effect) is immediate, affects the entire population, and occurs over the entire period considered. Thus the cumulative effect of the predator over the cohort lifetime of the prey can be very significant. Density reduction (the lethal effect), in contrast, occurs gradually over time and is transmitted only in proportion to the individuals removed and not the entire prey population. Thus the foraging reduction could have a significant effect even when the lethal effect is large.
In this study, we present results of an experiment examining the contribution of the nonlethal effect of a predator over a range of “effect space” (hereafter termed the predator–prey effect space) of predator–prey interactions; i.e., over a range of lethal (i.e., density) and nonlethal (i.e., trait-mediated) effects of a predator. We view these different combinations as representing different potential predator–prey interactions. They could, for example, represent different predators and prey, the same predators and prey at different sizes, or predator–prey interactions in different habitats where interaction magnitudes might differ.
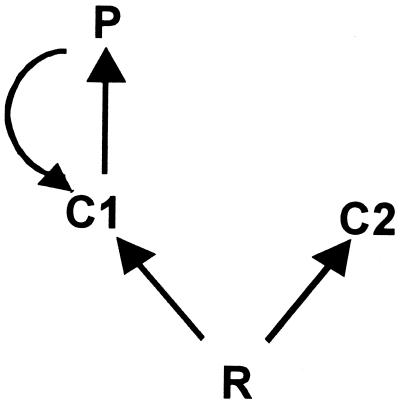
We used the simple food web in Fig. 1 as our model system and examined the indirect effects of the predator (P) through its prey (C1) on resources (R) and a competitor (C2). This food web allows us to examine both trophic cascades and exploitative competition, which are common indirect interactions known to influence communities. The predator in this food web has two effects on C1. First, the lethal effect, in which the predator preys on C1 and thus reduces its density. Second, the nonlethal effect, in which the predator induces trait-changes of surviving C1 individuals. In particular, the trait (or collection of traits) we consider represents a reduction in foraging activity of C1, a widespread response of prey to predators (10, 11). Both effects of the predator (reduction in numbers of C1 and per capita foraging rates of C1) can cause an increase in density of R and therefore more resources available to C2. The predator's effect on C1 foraging rate also permits C2 to acquire a larger fraction of the available resources independent of changes in density of R. Hence, the predator's presence can have a positive indirect effect on C2 through its effect on R density and how R is partitioned. We examine this food web with C2 density held low, thus asking how the induced reduction in foraging activity of C1 will affect the community in the absence of reciprocal effects from C2.
Figure 1.
The model food web. Straight arrows represent consumer–resource interactions and point in the direction of energy flow; the curved arrow represents a predator-induced change in prey traits that are associated with a reduction in foraging activity. The straight and curved arrows thus represent the lethal and nonlethal effect of the predator, respectively. In the experiment, C1, C2, and P were represented by small bullfrog tadpoles, large bullfrog tadpoles, and larval dragonflies, respectively. R were primarily periphyton and detritus.
Methods
We used a simple community in aquatic mesocosms to test whether the nonlethal predator effect can be influential over a wide range of predator–prey effect space. We examined the indirect effects of larval dragonfly predators (Anax sp., represented by P in Fig. 1) on large (second-year) larval bullfrogs (Rana catesbeiana, C2) through their lethal and nonlethal effects on small larval bullfrog prey (C1). Small bullfrog tadpoles react strongly to presence of Anax by reducing activity levels (19), whereas the large bullfrog tadpoles are much less vulnerable and react only weakly. We therefore can examine indirect effects of the predator on competitors (through vulnerable prey) without confounding effects of the predator on the competitors. Further, we used a low density of large bullfrog tadpoles to minimize their effects on resources and therefore permit a clearer differentiation of nonlethal and lethal effects of the predator through small bullfrog tadpoles.
Mesocosms mimicking small pond environments were constructed in cylindrical cattle watering tanks filled with 1,300 liters of well water and inoculated with phytoplankton, periphyton, and zooplankton from a local pond. Dry oak leaves (Quercus sp.) were added to each tank (300 g) as a substrate and to add physical complexity. We added 15 mg of nitrogen (in the form of NH4NO3), and 2.7 mg of phosphorus (KH2PO4) daily to support periphyton growth. Each tank was covered with 60% shade cloth to deter colonization by aquatic insects and other frogs and received four small predator cages constructed from slotted plastic drainpipe with ends enclosed by fiberglass window screening. Small bullfrog tadpoles (180; 10 ± 2.5 mg) hatched from several egg masses were added to each tank after a layer of periphyton developed on the tank walls and leaf surfaces.
Three levels of density reduction in small tadpoles were crossed with four levels of induced foraging reduction to effectively create 12 different combinations in the predator–prey effect space. The density reductions (no cue of predator presence) were established by manually removing 0%, 4%, and 25% of the small tadpoles with small nets once every 2 or 3 days. This exponential removal schedule led to final density reductions of 0%, 22%, and 83% over background mortality (16%). We isolated prey foraging reduction (no density reduction) by using different numbers of caged Anax (zero, one, two, and four) to which the small tadpoles react (due to chemical cues that diffuse out of the permeable cages). The 12 different combinations thus spanned a range of hypothetical predator–prey interactions, i.e., each density reduction-caged Anax treatment combination represents different “predators” with differing magnitudes of lethal and nonlethal effects on the prey. In an additional (13th) treatment, hereafter termed the “lethal Anax” treatment, we added two (uncaged) Anax to tanks. All 13 treatments were randomly distributed in each of four spatial blocks.
Experimental manipulations were initiated after a 10-day period in which the small bullfrog tadpoles were allowed to acclimate and grew to an average mass of ≈140 mg (as ascertained from sampling). We then added five large bullfrog tadpoles (1.8 ± 0.25 g) to each tank, placed one final-instar Anax in zero, one, two, or four of the four predator cages, and added two (uncaged) penultimate-instar Anax (≈0.63 g) to the lethal Anax treatment. Caged Anax were fed 3–5 small bullfrog tadpoles (totaling ≈350 mg of tadpoles per Anax) three times weekly. Nutrients originating from caged Anax do not contribute significantly to the tadpole growth rates and hence do not confound the results presented here (S.D.P., unpublished data).
We performed behavioral observations of tadpoles on seven occasions; during the afternoon of days 5, 7, 9, 21, and 23, and during the night (1–4 a.m.) of days 9 and 21. We slowly circled each tank and recorded the number of tadpoles above the tank floor (i.e., on or near the tank sides) and whether these tadpoles were active (swimming or feeding). This was done from four to six times over a 2- or 3-h period and the data for each tank were averaged for each measurement period. Bullfrog tadpoles generally react to Anax by spending more time on the tank floor and becoming less active (19). Note, these behavioral responses do not represent a direct assessment of the magnitude of foraging reduction of the tadpoles. This assessment would require a more detailed understanding of how behavioral traits such as percent of time spent active relate to the foragers ability to garner food. Rather, the behavioral observations were made to ensure that the small tadpoles indeed perceived, and responded to, the caged Anax predators, and to gain a qualitative estimate of how different numbers of caged Anax affected the relative tadpole foraging effort.
Results
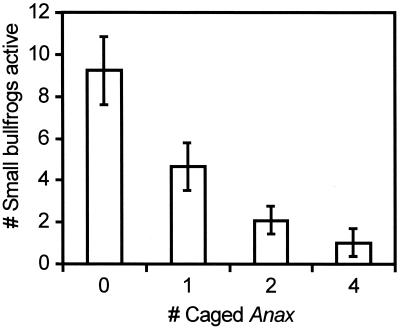
The presence of Anax had a large negative effect on tadpole traits related to foraging effort, and this effect was a function of the density of caged Anax (Fig. 2). For example, on day 5, there was a 34%, 52%, and 64% reduction in the number of small tadpoles on the tank sides (where they are presumably more vulnerable than on the tank floor) with one, two, and four caged Anax, respectively. Further, the tadpoles on the tank sides were ≈27%, 62%, and 82% less active with one, two, and four caged Anax, respectively, than in the control (where 25% ± 3.1% were active). Equally strong effects were also observed on days 7, 9, 20, and 22. There was only a weak effect of caged Anax at night (≈10% changes in tadpole position and activity levels with four caged Anax). The small bullfrogs reacted slightly more to the lethal Anax (two uncaged Anax) than to four caged Anax. In summary, the behavioral patterns of the small tadpoles suggest that lethal and caged Anax had a negative effect on the foraging effort of the small bullfrog tadpoles throughout the experiment, that the effect was stronger with more caged Anax, and that four caged Anax had roughly the same effect as two lethal Anax. In contrast, the caged Anax had no effect on the position and activity levels of the large bullfrog tadpoles.
Figure 2.
Behavioral response of small bullfrog tadpoles to caged Anax. The number of tadpoles active (swimming or feeding) was lower in tanks with caged Anax throughout the experiment (data presented here were collected early in the experiment on July 7).
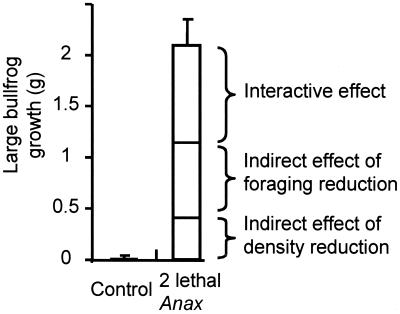
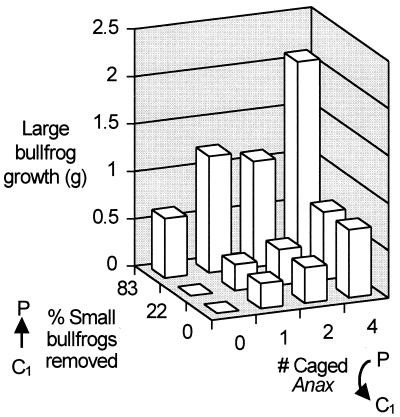
Large bullfrog tadpoles gained considerably more mass (2.1 ± 0.26 g, average gain per individual ± SE) in the lethal Anax treatment than in no-predator controls (0.009 ± 0.034 g) (Fig. 3). This difference is presumably due to the indirect effects of Anax both through changes in density and behavior of small tadpoles. When the nonlethal effect was isolated, increased number of caged Anax (from zero to one, two, and four caged Anax) had a large, monotonically increasing, effect on large tadpole growth (Fig. 4). When the density effect on small tadpoles was isolated, the low (22%) reduction had no apparent impact on large tadpole growth, whereas the high (81%) reduction had a large positive effect on large tadpole growth (Fig. 4).
Figure 3.
Growth (final minus initial mass) of large bullfrog tadpoles with and without two lethal Anax. Additional treatments allowed us to decompose the net indirect effect of the Anax into contributions due to foraging reduction, density reduction, and an interaction between these two effects (see text).
Figure 4.
Average large bullfrog tadpole growth (g, final minus initial mass) as a function of small bullfrog tadpole (C1) density reduction (using nets) and foraging rate reduction (using caged Anax). For clarity, error bars are not included. The average SE = 0.1 g.
Comparison of large bullfrog tadpole growth in the lethal Anax treatment with the manual removal treatments suggest that the Anax-induced reduction in small tadpole foraging rate contributed substantially to the total indirect effect of Anax on the large tadpoles. The two lethal Anax killed ≈50% of the small bullfrog tadpoles. If we interpolate from the isolated removal (no predator cue) treatments, this removal rate should lead to an ≈0.3–0.5 g increase in large bullfrog growth (a conservatively high estimate based on linear interpolation). The actual effect of lethal Anax on the large bullfrogs (2.1 g, Fig. 3) therefore greatly exceeded that predicted from the isolated effect of density reduction: the net indirect effect of the lethal Anax on large tadpole growth was from 4 to 7 times greater than that predicted from the effects of density reduction alone.
Further, the indirect effect of lethal Anax on large bullfrog tadpoles (causing a 2.1 g increase in mass) was ≈50–100% greater than that expected from the sum of isolated indirect effects due to density reduction (≈0.3–0.5 g) and foraging reduction [because the two lethal Anax had about the same effect on tadpole traits as four caged Anax, we would expect that the nonlethal effect of the Anax (in the lethal Anax treatment) to be ≈0.8 g (Fig. 4)]. We suspect two potential causes for this disparity (i.e., interaction). First, tadpoles may gain mass at a faster rate as they grow larger. Thus the two mechanisms (i.e., density and foraging reduction of small tadpoles) that cause an increase in large bullfrog tadpole growth rate could interact to cause a greater net effect than the sum of their effects in isolation. Second, both the removal and trait modification of the small tadpoles represent a decrease in herbivory on the resources. If resources respond nonlinearly to this decrease, then the increase in resources may be greater than the sum of the increases caused by density and foraging reduction of small tadpoles in isolation.
Results of the 12 different hypothetical predator–prey interactions indicated that the nonlethal effect of the predator contributed substantially to the net indirect effect of the predator over a large range of the predator–prey effect space (Fig. 5). To relate the hypothetical (i.e., experimentally constructed) predator–prey interactions to real predator–prey interactions, we expressed them in terms of the lethal Anax–small tadpole interaction magnitude. For example, because two free Anax had a slightly larger effect on tadpole behavior than four caged Anax, we can estimate that the effect of one, two, and four caged Anax is approximately that of one-half, one, and two lethal (uncaged) Anax. Further, the two Anax removed 50% of the small tadpoles, so we can roughly estimate that our removal levels of 22% and 83% correspond to slightly lower than one and slightly greater than three free Anax, respectively. Clearly this procedure provides rough estimates; however, we use this exercise only to provide a qualitative understanding of the relative contribution of the nonlethal effect of predators.
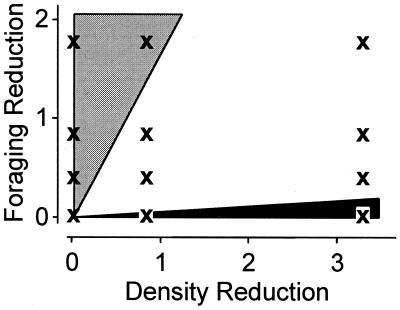
Figure 5.
Representation in predator–prey effect space of the contribution of foraging reduction and density reduction to the total indirect effect of the predator on large bullfrog tadpoles. Units for both density and foraging reduction represent one lethal Anax in the experimental tank environment and were calibrated by extrapolating from the effect of Anax in the lethal Anax treatment on density and behavior of the small tadpoles. Xs correspond to the 12 combinations of density reduction and foraging reduction probed by the 3 × 4 factorial design. The light and dark shaded regions represent regions in predator–prey effect space where foraging reduction and density reduction represent >95% of the total effect of the predator, respectively, whereas the nonshaded region represents parameter combinations for which both effects contribute >5%. We derived these regions by examining the impact of foraging reduction and density reduction in the factorial design treatments (Fig. 4).
We plotted the relative impact of the density and nonlethal effects of Anax on large bullfrog tadpole growth by divided the predator–prey effect space into sections where either the density effect or the induced trait effect represented ≥95% of the total effect of the predator on large tadpoles, or a third section where both effects contributed >5% (Fig. 5). The density effect contributed ≥95% of the total effect only when there was no trait modification and the density reduction rate was relatively large (at three, but not one, lethal Anax equivalent). In contrast, there is a larger region of predator–prey effect space where effects due to trait modification represented ≥95% of the total indirect effect. In fact, when the nonlethal effect was relatively low (equivalent to approximately one-half lethal Anax), it contributed substantially to the total indirect effect of the predator even at high density-reduction rates (equivalent to approximately three to four lethal Anax).
Discussion
This experiment demonstrated that the nonlethal effect of a predator can contribute substantially to its net indirect effect over a large range of predator–prey effect space (Figs. 2 and 5). Our data suggest that the lethal effect (i.e., the density reduction) dominated the indirect effect of the predator on large bullfrog tadpoles over only a very small range of predator–prey effect space, whereas foraging reduction dominated a much larger range of the predator–prey effect space. This was the case even at high predation (removal) rates. Further, the net indirect effect of the uncaged “lethal” Anax was far greater (4 to 7 times) than that predicted from density effects alone due to the nonlethal effect and an interaction between the nonlethal and lethal effects (Fig. 3).
Our results make clear that phenotypic responses of prey likely play a large role in determining the consequences of species interactions. Other investigations have suggested that TMIIs are likely important in diverse systems (refs. 13–22 and references therein). These studies have primarily examined the nonlethal effect of a predator by either examining the nonlethal effect isolated from the density effect or by investigating predator–prey interactions in size ranges where predation is negligible (e.g., refs. 23 and 24). Here we show that the nonlethal effect can be influential not only when density effects are low, but over a large range of induced foraging rate reduction–predation rate combinations, including those where predation rates are high.
Although perhaps counterintuitive, there are two straightforward reasons behind these results. First, the nonlethal effects occur independent of lethal effects; i.e., TMIIs arise through potential prey that remain in the system, whereas DMIIs arise due to the removal of prey from the system. Therefore an induced reduction in foraging by X% causes an X% reduction in energy gained by the prey population and an X% reduction in the prey's effect on the resources, regardless of predation rates. Second, nonlethal effects occur over the duration of each prey's lifetime. Thus the nonlethal effects are transmitted from the predator to the resources (and therefore competitors) even through prey that are ultimately killed. For example, if prey at age X are killed by a predator, the indirect effects of the predator through induced trait changes in the prey to time X still have affected the system. These two reasons suggest that TMIIs can be important at high, as well as low, predation rates.
Our results have far-reaching implications to the study of ecological communities. Indirect interactions that arise from predator-induced trait changes (phenotypic plasticity) differ from those that arise from density changes in fundamental ways. First, it is likely that indirect interactions resulting from a predator's effect on density and traits of prey will exhibit markedly different density dependencies. For example, a few predators may have a strong effect on prey traits (and hence a large effect on the net foraging impact of the prey) but little effect on prey density, whereas many predators will have a proportionately stronger effect on prey densities. Second, indirect interactions arising from changes in traits of a species imply that the interaction magnitudes between two species are dependent on the background species assemblage. Thus we cannot predict the dynamics of complex assemblages on the basis of interactions of small subsets of species in isolation (13–18). Rather, models must recognize that interaction strengths between any two species change dynamically with the density of other species in the system. Recent theoretical work shows that inclusion of higher-order terms that would be required to describe our short-term results can have a profound influence on predictions of models describing long-term population dynamics (13, 25–29).
In conclusion, experimentally isolating the separate components of the indirect effects of a predator and examining the predator–prey phase effect of predator–prey interactions demonstrated that a predator-induced modification in prey traits can contribute substantially to the net indirect effect of a predator even when there are large density effects. The typical representation of ecological communities, in which trait modification is tacitly assumed to be inconsequential, is thus inadequate to describe the dynamics of the community examined here. An abundance of recent empirical evidence demonstrates that the reaction of the anuran larvae to their predator that underlies our results is exhibited in many species from diverse taxa. This demonstration suggests that our results likely apply to a broad range of ecological communities, and that many effects traditionally attributed to predators eating prey may actually be due to predators inducing changes in the traits of their prey.
Acknowledgments
We thank Robert Rommel and Chad Walasek for assistance in conducting the experiment. We thank P. Abrams, L. Curran, D. Goldberg, G. Kling, M. Holyoak, O. Schmitz, J. Shurin, J. VanBuskirk, and J. Vandermeer for helpful comments on the manuscript. This work was funded by National Science Foundation Grant DEB 9615523.
Abbreviations
- DMII
density-mediated indirect interactions
- TMII
trait-mediated indirect interactions
- P
predator
- C1
prey
- C2
competitor
- R
resources
References
- 1.Brooks J L, Dodson S I. Science. 1965;150:28–35. doi: 10.1126/science.150.3692.28. [DOI] [PubMed] [Google Scholar]
- 2.Paine R T. Am Nat. 1966;100:65–75. [Google Scholar]
- 3.Estes J A, Palmisan J F. Science. 1974;185:1058–1060. doi: 10.1126/science.185.4156.1058. [DOI] [PubMed] [Google Scholar]
- 4.Oksanen L, Fretwell S, Arruda J, Niemala P. Am Nat. 1981;118:240–261. [Google Scholar]
- 5.Carpenter S R, Kitchell J F, Hodgson J R, Cochran P A, Elser J J, Elser M M, Lodge D M, Kretchmer D, He X, Vonende C N. Ecology. 1987;68:1863–1876. doi: 10.2307/1939878. [DOI] [PubMed] [Google Scholar]
- 6.Schoener T W. In: Mutualisms and Community Organization in Ecological Communities. Kawanabe H, Cohen J E, Iwasaki K, editors. Oxford: Oxford Univ. Press; 1993. pp. 365–411. [Google Scholar]
- 7.Menge B A. Ecol Monogr. 1994;65:21–74. [Google Scholar]
- 8.Wootton J T. Annu Rev Ecol Syst. 1994;25:443–466. [Google Scholar]
- 9.Leibold M A, Chase J M, Shurin J B, Downing A L. Annu Rev Ecol Syst. 1997;28:467–494. [Google Scholar]
- 10.Lima S L. Stress Behav. 1998;27:215–290. [Google Scholar]
- 11.Lima S L, Dill L M. Can J Zool. 1990;68:619–640. [Google Scholar]
- 12.Tollrian R, Harvell C D, editors. The Ecology and Evolution of Inducible Defenses. Princeton: Princeton Univ. Press; 1999. [Google Scholar]
- 13.Abrams P A. In: Predation: Direct and Indirect Impacts on Aquatic Communities. Kerfoot W C, Sih A, editors. Hanover, NH: Univ. Press of New England; 1987. pp. 38–54. [Google Scholar]
- 14.Sih A. In: Predation: Direct and Indirect Impacts on Aquatic Communities. Kerfoot W C, Sih A, editors. Hanover, NH: Univ. Press of New England; 1987. pp. 203–224. [Google Scholar]
- 15.Werner E E. Am Nat. 1992;140:S5–S32. [Google Scholar]
- 16.Wootton J T. Am Nat. 1993;141:71–98. [Google Scholar]
- 17.Abrams P A, Menge B A, Mittelbach G G, Spiller D, Yodzis P. In: Food Webs: Integration of Patterns and Dynamics. Polis G A, Winemiller K O, editors. New York: Chapman & Hall; 1996. pp. 371–395. [Google Scholar]
- 18.Schmitz O J. Am Nat. 1998;151:327–342. doi: 10.1086/286122. [DOI] [PubMed] [Google Scholar]
- 19.Peacor S P, Werner E E. Ecology. 2000;81:1998–2010. [Google Scholar]
- 20.Huang G, Sih A. Oecologia. 1991;85:530–536. doi: 10.1007/BF00323765. [DOI] [PubMed] [Google Scholar]
- 21.Wissinger S, McGrady J. Ecology. 1993;74:207–218. [Google Scholar]
- 22.Diehl S, Cooper S D, Kratz K W, Nisbet R M, Roll S K, Wiseman S W, Jenkins T M., Jr Am Nat. 2000;156:293–313. [Google Scholar]
- 23.Beckerman A P, Uriarte M, Schmitz O J. Proc Natl Acad Sci USA. 1997;94:10735–10738. doi: 10.1073/pnas.94.20.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner A T, Mittelbach G G. Ecology. 1990;71:2241–2254. [Google Scholar]
- 25.Abrams P A. In: Mutualisms and Community Organization in Ecological Communities. Kawanabe H, Cohen J E, Iwasaki K, editors. Oxford: Oxford Univ. Press; 1993. pp. 255–279. [Google Scholar]
- 26.Abrams P A. Am Nat. 1995;146:112–134. [Google Scholar]
- 27.Fryxell J M, Lundberg P. Individual Behavior and Community Dynamics. New York: Chapman & Hall; 1998. [Google Scholar]
- 28.Krivan V. Theor Popul Biol. 1998;53:131–142. doi: 10.1006/tpbi.1998.1351. [DOI] [PubMed] [Google Scholar]
- 29.Vandermeer J, Maruca S. Theor Popul Biol. 1998;54:38–43. doi: 10.1006/tpbi.1997.1357. [DOI] [PubMed] [Google Scholar]