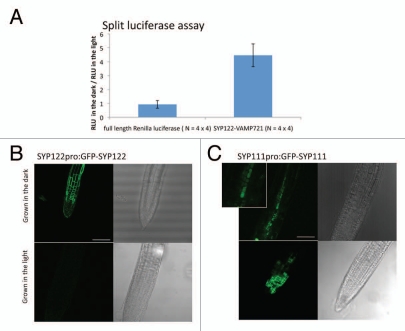
Figure 1.
Changes in expression, localization and interaction of SNARE proteins in Arabidopsis cells in the dark. (A) Enhancement of the SYP122-VAMP721 interaction in the split luciferase complementation assay. Recombinant SYP122 and VAMP721 proteins, genetically fused to the N- and C-terminal fragment of Renilla luciferase respectively, were expressed by constitutive promoters in Arabidopsis protoplasts. The protoplasts were then incubated in the light (100 µmol/m2/s) or the dark for 16 h before measuring relative luminescence units (RLU). As a control, full length Renilla luciferase was expressed. An average of the RLUs of four independent transformations in a 96-well plate incubated in the dark was divided by an average of the RLUs of four independent transformations in another 96-well plate incubated in the light. The results from four independent experiments are presented with the standard errors. The changes of the RLUs in SYP122-VAMP721 (4.46-fold) are significantly (p = 0.012) higher than that in full-length Renilla luciferase (0.93-fold). (B) GFP-SYP122 is expressed in the root tip in the dark. Seedlings of transgenic Arabidopsis expressing GFP-SYP122 by the syp122 promoter (SYP122PRO:GFP-SYP122) were grown in the dark (24 h) or light (100 µmol/m2/s, 16 h light and 8 h dark) for five days. Transmission images of root tips are shown on the right side of fluorescence images for reference. Bar = 500 µm. (C) GFP-SYP111/KNOLLE is localized in the vacuoles, instead of division planes, in the dark. Seedlings of transgenic Arabidopsis expressing GFP-SYP111 by the syp111 promoter (SYP111PRO:GFP-SYP111) were grown in the dark (24 h) or light (100 µmol/m2/s, 16 h light and 8 h dark) for five days. An enlarged fluorescence image for the dark-grown seedling is also shown to clarify the vacuole accumulation of GFP-SYP111. Bar = 500 µm.