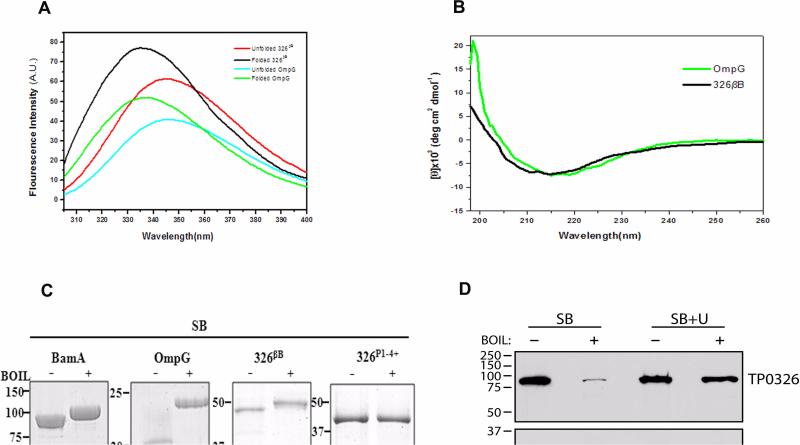
Figure 2. The C-terminal portion of TP0326 forms a β-barrel.
(A) Tryptophan fluorescence emission spectra of unfolded 326βB and OmpG and folded forms in 0.5% DDM and 1% OG micelles, respectively. (B) CD spectra of folded 326βB (5 μM) and OmpG (2.6 μM) in DDM and OG buffer, respectively. (C) Heat-modifiability of folded E. coli BamA, OmpG, 326βB, and 326P1-4+. Proteins were stained with GelCode® Blue following SDS-PAGE with (+) or without (-) boiling in sample buffer (SB). (D) T. pallidum samples were dissolved in SB without (SB) or with (SB + U) 8M urea prior to SDS-PAGE with (+) or without (-) boiling and immunoblotted against POTRA or TroA (loading control) antiserum. MW standards (kDA) for SDS-PAGE and immunoblot analyses are on the left of each panel.