Abstract
Rationale
Although studies suggest that stress is an important reason for relapse in alcoholics, few controlled studies have been conducted to examine this assumption. Evidence of stress-potentiated drinking would substantiate this clinical observation and would contribute to the development of a model that would be valuable to alcohol treatment research.
Objectives
The hypothesis was tested that an acute psychosocial stressor, the Trier Social Stress Test (TSST), increases alcohol consumption in non-treatment-seeking alcoholics.
Methods
Seventy-nine alcohol-dependent participants (40 women) were randomly assigned to receive the TSST or a no-stress condition. Immediately afterward, all participants received an initial dose of their preferred alcoholic beverage to achieve a target blood alcohol concentration of 0.03 g/dl (to prime subsequent drinking in the laboratory). Participants then participated in a mock taste test of two glasses of beer. Primary dependent measures were whether s/he drank all of the beer available (yes/no) and total amount of beer consumed (milliliters).
Results
Stressed participants were twice as likely as non-stressed participants to drink all of the beer available, a significant effect. Although the stressed group drank more milliliters than the non-stressed group, this effect failed to reach significance, likely due to ceiling effects. There were no significant stress group × gender effects on either outcome.
Conclusions
This study supports that stress-potentiated drinking is valid and can be modeled in a clinical laboratory setting.
Keywords: Alcohol, Relapse, Cortisol, Stress, Gender, Model
Stress is broadly defined as a process by which a stimulus elicits an emotional, behavorial, and/or physiologic response (Ice and James 2007). That experiences interpreted as stressful can induce drinking is implicit in theories regarding the development and maintenance of alcohol use disorders (Conger 1951; Sayette 1993; Sher and Levenson 1982) and relapse (Marlatt and Gordon 1985; Zywiak et al. 1996). Several clinical studies report that relapse is attributed to stressful life events and/or one’s ability to cope with stress (Brown et al. 1995; Levy 2008; Vuchinich and Tucker 1996). Not surprisingly, most evidence-based treatments for alcohol dependence include stress-coping and mood management elements to help treatment seekers maintain sobriety (Marlatt and Gordon 1985; Monti et al. 2002; Vieten et al. 2010).
Although there is substantial clinical and anecdotal support for stress-induced relapse, determining conclusively that stress causes drinking is a challenge. To do so, randomized controlled studies are needed where individuals are exposed (or not) to a stress-inducing stimulus (a stressor), and subsequent drinking is measured. If more participants drink or if more alcohol is consumed following the stressor, the conclusion can be made that the stressor is the cause. If the outcome of interest is relapse (which is arguably the most ecologically relevant construct), participants must be alcoholics attempting abstinence, and such studies are not ethically tenable. In short, a study with the necessary methodological rigor and ecological relevance yet with an appropriate risk/benefit ratio may not be possible. Of the three controlled studies conducted to date, all have focused on stress-induced drinking, not relapse.
Higgins and Marlatt (1973) randomized 20 alcohol-dependent men and 20 non-dependent social drinkers to either a high psychological stress condition (anticipation of a painful electric shock) or a low stress condition (anticipation of a benign electric stimulation); the threat of the shock was the stressor—no shock was actually delivered. Following this information, participants were asked to engage in an alcohol taste test by rating three glasses of different alcoholic beverages on several dimensions. The primary outcome variable was the amount of alcohol consumed (milliliters); the “taste test” deception was used to diminish demand effects. As expected, alcoholics consumed more alcohol than social drinkers. However, the amount of alcohol consumed—regardless of alcohol dependence—did not differ by stress condition. Unfortunately, the stress manipulation lacked internal validity, as ratings of anxiety were not higher in the high stress vs. low stress condition, and so no conclusions could be drawn about stress-induced drinking.
Miller and colleagues (1974) tested a small sample of male alcohol-dependent (n=8) and non-problem drinkers (n=8) using a within-subjects design. Following a stressful condition (confrontational social scenarios with discouraging feedback and criticism) and a control condition (conversation about spare time activities), presented on separate testing days, participants could bar press to receive a small dose of alcohol. The primary outcome variables were number of bar presses made and the resulting volume of alcohol consumed. The results indicated that the stress manipulation was valid, as evidenced by increased pulse rate following the stress vs. no-stress condition. Alcoholics but not social drinkers showed increased lever pressing following the stress vs. no-stress conditions, and consequently, a greater amount of alcohol was consumed. The authors reported that all eight alcoholics increased their responding under the stress vs. no-stress condition, whereas the majority of social drinkers decreased their rates of responding under stress.
More recently, Pratt and Davidson (2009) used a within-subjects design and a relatively large sample (N=74, 22 were women) of non-treatment-seeking alcoholics. The Paced Auditory Serial Addition Test (Gronwall 1977) was the stressor, and a control condition of equal length was included. Participants completed the two sessions on separate days in counterbalanced order. Salivary cortisol was collected to confirm the validity of the stress manipulation. Alcohol consumption was assessed with a cue availability paradigm, whereby participants gained access to a glass of beer behind a locked window at a computer signal. Researchers assessed three primary drinking outcomes: (1) latency to open the window following the signal (to index motivation to drink), (2) amount of alcohol consumed, and (3) alcohol craving following both the stress and the neutral conditions. Change in cortisol confirmed the internal validity of the stress manipulation. Although the authors did not report whether the effect of the stressor on the three alcohol outcomes was significant, group means were provided and suggested an effect of stress was unlikely. For example, amount of alcohol consumed following the neutral vs. stress condition was 2.08 vs. 2.19 oz, respectively; mean values for latency and craving were similarly close. Thus, the stressor, while successfully inducing a stress response, did not appear to induce drinking or greater urge to drink.
In summary, evidence is lacking that stress induces drinking in alcoholics, albeit only three controlled studies have been conducted, only one of which included women. The present study was conducted to test the hypothesis that an acute psychosocial stressor potentiates drinking in non-treatment-seeking alcoholics. The study employed standardized and validated methods of stress induction and drinking for clinical laboratory settings. Objective and subjective measures of stress reactivity were collected to confirm the internal validity of the stress manipulation (a between groups variable), and an equal number of men and women were enrolled to explore the effect of gender on stress reactivity and stress-potentiated drinking.
Materials and methods
Participants
Participants (21–65 years) were recruited via community advertisements. Only non-treatment-seeking alcoholics were included in the study in accordance with ethical guidelines regarding providing alcohol to individuals attempting to abstain (National Advisory Council on Alcohol Abuse and Alcoholism 2005). Eligibility for the study was initially determined with a telephone screening, which included an assessment of quantity and frequency of alcohol use, dependence symptoms, alcohol treatment history, and interest in seeking treatment for alcohol problems (exclusionary). Because the laboratory challenge involved drinking beer, individuals who did not drink beer at least once per month were excluded. Preliminarily eligible callers were scheduled for an in-person visit.
Informed consent and assessment
Informed consent was obtained when the individual arrived at the in-person visit. The informed consent document, approved by the university’s institutional review board, provided explicit information regarding the procedures of the study, but deception was necessary regarding the challenge procedure to reduce demand effects. Participants were informed that they would randomly be selected (50% chance) to complete a “behavioral measure of how outgoing you are and a mental math test to help us collect information about your working memory, both of which tell us something about your personality,” which was the cover story for the stress induction procedure. An additional deception was employed to avoid alerting participants to the fact that their subsequent alcohol consumption would be measured. Participants were told that following the personality test, they would consume a small amount of their preferred alcoholic beverage, then they would be asked to taste and rate two glasses of beer and that the purpose of the study was to examine the effects of personality on ability to detect subtle differences in flavors.
A battery of psychometrically sound self-report instruments was completed by consenting individuals, including the Alcohol Dependence Scale (Skinner and Allen 1982), State/Trait Anxiety Inventory (Spielberger 1983), Anxiety Sensitivity Index (Reiss et al. 1986), Beck Depression Inventory-II (Beck et al. 1996; Beck et al. 1961), Barrett Impulsiveness Scale II (Patton et al. 1995), and the Drinking Motives Questionnaire (Cooper et al. 1992). The MINI (Sheehan et al. 1998) was conducted by clinical interview to confirm a diagnosis of alcohol dependence and rule out exclusionary Axis I disorders, and the Timeline Followback (Sobell et al. 1992) was administered to assess quantity and frequency of alcohol consumption over the past 30 days. Eligible participants met diagnostic criteria for current alcohol dependence and drank at least 14 drinks (women) or 20 drinks (men) per week. Participants were excluded if they met criteria for other current major Axis I disorders, including current dependence on other drugs (excepting nicotine), were taking any psychotropic medications or medications known to affect the hypothalamic pituitary adrenal (HPA) axis, had a body mass index >35, or had received prior medical alcohol detoxification.
Eligible participants were scheduled for the challenge visit at the end of the in-person visit. Women were scheduled to complete the challenge during the follicular phase (days 1–10 following menses), when estradiol and progesterone are relatively low. Use of oral contraception was not exclusionary. All participants were instructed that a urine drug test and alcohol breath test (and pregnancy test for women) would be administered on the day of the challenge, and all tests must be negative to proceed with the challenge (and to receive compensation for participation). They were further instructed to abstain from alcohol for 48 h prior to the scheduled challenge visit, abstain from caffeinated beverages on the day of the challenge, and fast for 4 h prior to the challenge.
Individuals were recruited from August 2008 to March 2010; 462 callers completed the telephone screening, and 115 progressed to the in-person visit. Of those individuals, 94 were eligible and 79 (40 women) completed the challenge. Of the 15 individuals who did not complete the challenge visit, seven were non-compliant with prechallenge requirements (e.g. abstinence from drugs and alcohol), six failed to schedule the challenge visit within 2 months of eligibility, and two (one from each stress group) withdrew consent during the challenge procedure.
Laboratory challenge
Schedule of procedures
The challenge visit occurred on average 14 days (range, 2–54) after the initial in-person interview, and all participants were tested individually. Participants arrived at the university’s Clinical and Translational Research Center (CTRC) at 4:00 pm, at which time compliance with pre-challenge instructions was assessed. The Clinical Institute Withdrawal Assessment of Alcohol Scale (Sullivan et al. 1989) was administered to ensure the absence of marked alcohol withdrawal (score of eight or less). At 4:15 pm, an indwelling catheter was placed in the non-dominant arm in order to obtain blood samples used to measure stress reactivity. From 5:00 pm to 5:15 pm, participants either underwent the stressor (described below) or the no-stress control condition (reading a travel magazine alone for 15 min).
To prime voluntary alcohol consumption, participants were given their preferred alcoholic beverage in a dose calculated to produce a maximum blood alcohol concentration of 0.03 g/dl (based on tables produced by Watson 1989). Participants were instructed to sip the drink evenly to finish it in 10 min (from 5:20 pm to 5:30 pm). This procedure has been reliably used to induce craving and/or subsequent drinking in alcoholics in clinical laboratory settings (Anton et al. 2004; Davidson et al. 2003; O’Malley et al. 2002).
From 5:45 pm to 6:00 pm, participants completed the taste test procedure (described below), and subsequent stress and breath alcohol readings were taken until 7:00 pm, when the catheter was removed. Breath alcohol was assessed again at 7:15 pm, at which point those participants with a blood alcohol concentration (BAC) less than 0.03 g/dl were provided a feedback report of the results from questionnaires administered during the previous in-person interview visit, including a personality profile to corroborate the study’s reported purpose (effects of personality on alcohol discrimination) and quantity and frequency of alcohol use and information about available alcohol treatment services. Participants with BAC≥0.03 g/dl remained in the laboratory until BAC dropped below 0.03 g/dl. All participants were compensated $200 and transported home by taxi.
Stress manipulation
The Trier Social Stress Test (TSST; Kirschbaum et al. 1993) is an acute psychosocial stressor with both a social evaluative component (public speaking about oneself) and a performance component (mental math). The TSST is considered the gold standard for eliciting an HPA axis response in a laboratory setting (Dickerson and Kemeny 2004) and has been consistently shown to induce a marked stress response in both men and women (Kudielka et al. 2007). The stress induction procedure is described elsewhere (Thomas et al. 2011). Briefly, at 5:00 pm participants randomized to the TSST were instructed that they would complete a behavioral personality test in front of three individuals with expertise in assessing body language and behavior. Participants completed a 5-min preparation phase and then were escorted to a separate room to perform a 5-min speech task and a 5-min serial subtraction task in the presence of an audience. Audience members were three confederates in white lab coats who were trained to remain stoic during the participant’s presentation. Following the serial subtract task, the research technician escorted the participant back to the original room. Participants randomized to receive the no-stress control condition remained in the original room and read a travel magazine for 15 min.
Taste test
The taste test procedure was a modified version of the procedure used by Marlatt and colleagues (Caudill and Marlatt 1975; Higgins and Marlatt 1973) and afforded a covert assessment of alcohol consumption. All participants were given two glasses (labeled A and B), both of which contained equal parts of cold Budweiser and O’Douls non-alcoholic beer. Non-alcoholic beer was mixed with alcoholic beer to minimize the peak blood alcohol concentration if the participant consumed both glasses (355 ml per glass; 710 ml or 24 oz total). With this beverage mixture, the estimated peak BAC would not exceed 0.05 g/dl for most participants, and thus the BAC would drop below 0.03 g/dl (required for discharge from the challenge) within 1–2 h.
Participants were instructed that they had 15 min to taste each glass to determine whether glass A and glass B contained identical or different beers and to rate additional qualities about each beer. Participants were told to drink whatever amount necessary to make an accurate decision and that a correct response would earn a $15 bonus to their base compensation.
The experimenter provided the two glasses of beer plus an 8-oz glass of water (to control for general thirst, de Wit et al. 2003) and left the room. At the end of 15 min, the experimenter returned and collected the glasses and the participant’s rating sheet. The taste test procedure was video recorded to examine participants’ drinking behavior (e.g., latency to first sip). All participants were told that their decision about the two beers was correct, and all received the bonus payment.
Laboratory assessments
Stress reactivity
Response to the stress manipulation was measured with subjective and objective measures. Subjective distress was assessed using a 10-point scale from 1=not at all stressed to 10=severely stressed. Objective indices of stress reactivity were mean arterial pressure (millimeters of Mercury) and serum cortisol (micrograms per deciliter). Each of these outcomes was collected nine times, twice prior to the stress manipulation and seven times post-stressor (at 0, 15, 45, 60, 75, 90, and 105 min).
Blood samples for cortisol were collected in iced EDTA tubes; plasma was separated from cells by centrifugation, and the serum sample was frozen at −70°C until thawed for assay. Cortisol was assayed using the ADVIA Centaur XP immunoassay system (Siemens Healthcare Diagnostics, Flanders, NJ). Functional sensitivity was 0.2 µg/dl, and intra-assay cv was 2.15% at 44 µg/dl.
Blood alcohol concentration
Breath alcohol (Alco-Sensor IV, Intoximeters, St. Louis, MO) was obtained to index blood alcohol concentration. BAC was collected to examine whether the alcohol priming dose induced the target level (0.03 g/dl) and to ensure that no participant was dismissed from the challenge with BAC≥0.03 g/dl. BAC was assessed at arrival (4:05 pm, required to be 0.00 g/dl to participate in the challenge), following the alcohol priming but prior to the taste test (5:44 pm), and five times following the taste test (0, 15, 30, 60, and 75 min).
Craving
The Alcohol Urge Questionnaire (AUQ; Bohn et al. 1995) was used to measure urge to drink. The AUQ is a validated instrument with a range of 8–56 for assessing subjective experience of state craving (Drummond and Phillips 2002). It has a single factor structure and has been used in clinical laboratory studies to assess real-time measurement of alcohol craving (MacKillop 2006). It was administered five times—at baseline (arrival), after the stressor manipulation (5:15 pm), after the alcohol priming (5:30 pm), after the taste test (6:00 pm), and prior to dismissal (6:45 pm). No participant was dismissed from the challenge with elevated craving (as defined by last assessment being >30% above the baseline assessment).
Alcohol consumption
Two primary and three secondary measures were used to assess alcohol consumption from the taste test. Primary measures were probability of consuming all the beer provided and the amount of beer consumed (milliliters). Secondary measures were latency to first sip (seconds), median latency between sips (seconds), and the average amount consumed per sip (total milliliters consumed divided by number of sips).
Statistical analysis
Demographics, pre-challenge drinking, and psychological severity measures were analyzed with two (stress group) × two (gender) analysis of variance or logistic regression for dichotomous variables. Analyses characterizing the stress response across time (e.g., cortisol, mean arterial pressure, subjective distress) and craving over time (AUQ) were conducted as linear mixed models (SAS PROC MIXED) with an unstructured covariance matrix and stressor group and gender as independent variables. Breath alcohol outcomes following the priming dose of alcohol were analyzed with analysis of variance with stressor group and gender as factors, and single sample t tests to confirm that actual BAC did not differ significantly from target BAC (0.03 g/dl). Analyses of the two primary alcohol consumption variables (milliliters beer consumed and probability of drinking all the beer provided), and all secondary alcohol consumption variables were performed as analyses of covariance with drinks per day (from the Timeline Followback) as a covariate. Unadjusted means are reported unless otherwise specified.
Results
Participants
Demographics, alcohol use, preferred alcoholic beverage, and psychological assessment descriptive statistics are presented by stress group and gender in Table 1. One participant in the control group reported drinking amounts that were consistently >3 times the interquartile range; her drinking data were excluded from analyses.
Table 1.
Demographics, alcohol use, and psychological characteristics of participants
| No-stress | Stressor | Effects of stress group, gender, stress × gender |
|||
|---|---|---|---|---|---|
| Men | Women | Men | Women | ||
| N (total=79) | 19 | 20 | 20 | 20 | |
| Demographics | |||||
| Caucasian | 79% | 85% | 80% | 75% | ns |
| Age | 33 (9.4) | 35 (10.9) | 35 (11.1) | 35 (12.6) | ns |
| Employed fulltime | 58% | 30% | 45% | 35% | ns |
| Married | 5% | 15% | 20% | 10% | ns |
| Smoker | 47% | 45% | 65% | 30% | ns |
| College graduate | 32% | 30% | 35% | 30% | ns |
| Alcohol use | |||||
| Drinks per week | 87 (73.3) | 53 (38.0) | 61 (35.8) | 59 (47.3) | ns |
| Drinks per drinking day | 15 (11.2) | 10 (5.2) | 11 (5.0) | 11 (6.3) | ns |
| ADS [0–47] | 15 (6.6) | 16 (6.7) | 14 (5.4) | 16 (8.0) | ns |
| Age onset alcohol dependence | 23 (7.6) | 24 (5.1) | 24 (6.1) | 25 (9.7) | ns |
| Psychological assessment | |||||
| Trait anxiety, STAI [20–80] | 38 (12.4) | 39 (9.8) | 39 (9.8) | 48 (13.1) | Women > men |
| Anxiety sensitivity, AnxSI [0–64] | 17 (9.4) | 18 (7.6) | 19 (10.0) | 25 (11.4) | Stress > no-stress |
| Depressive symptoms, BDI [0–63] | 11 (7.9) | 11 (7.3) | 9 (7.7) | 17 (9.9) | Stress × gender |
| Impulsivity, BIS [30–120] | 68 (11.8) | 67 (8.27) | 65 (10.5) | 74 (9.5) | Stress × gender |
| Drinks to cope, DMQ [0–4] | 2.5 (0.6) | 2.5 (0.7) | 2.2 (0.6) | 2.7 (0.7) | ns |
| Alcohol craving, AUQ [8–56] | 26 (11.8) | 28 (14.8) | 30 (10.3) | 31 (15.8) | ns |
Brackets show each instrument’s minimum and maximum value. All effects reported p<0.05
ADS Alcohol Dependence Scale, STAI State Trait Anxiety Inventory, AnxSI Anxiety Sensitivity Index, BDI Beck Depression Inventory, BIS Barrett Impulsiveness Scale, DMQ Drinking Motives Questionnaire (Coping subscale), AUQ Alcohol Urge Questionnaire
Randomization (stressor vs. no-stress control) yielded equivalent groups on demographics and alcohol use. Participants had a mean age of 34 years (SD=11, range, 21–57), consumed 65 drinks per week, ten drinks per day, and 12 drinks per drinking day. There were no significant differences between stressor groups or gender on alcohol use or preferred alcoholic beverage. Percent of men and women whose preferred alcoholic beverage was beer was 62% vs. 53%, respectively. Alcohol dependence severity (M=15, SD=6.7) was in the moderate range. Alcohol use between the initial visit and challenge visit was not assessed, although participants were asked to report the date of their last drink in order to assess compliance with 48-h abstinence. Participants on average reported 3 days since their last drink, and there were no main or interaction effects of stress group or gender on number of days since last drink.
Significant differences between groups in some measures of psychological severity were revealed. Participants randomized to the stressor group reported higher anxiety sensitivity than those in the control group, F(1,75)=5.18, p<0.05. Women reported significantly higher depression severity (BDI) than men, F(1,75)=4.53, p<0.05, and there were significant stressor group × gender interactions for depression severity and impulsivity (BIS), both Fs(1,75)>4.60, p values<0.05. Women in the stressor group had higher mean scores than women in the control group on both of these measures, with no-stressor group differences in men. These variables were examined as potential covariates in analyses of stress reactivity and drinking but were dropped if their inclusion did not alter results.
Effect of the stressor
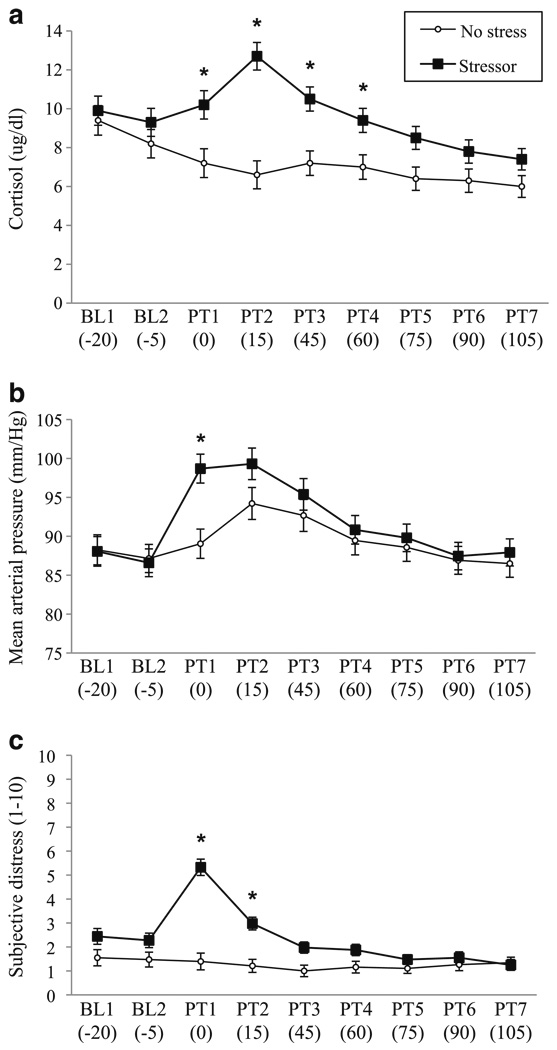
There was a significant stressor group × time effect for cortisol (Fig. 1a), mean arterial pressure (Fig. 1b), and subjective distress (Fig. 1c), all F values (8.74)>8.2, all p values<0.05. For each of these measures, the stressor and control groups were similar at the two baseline timepoints, and groups diverged (stressor > no-stress) following the stress manipulation.
Fig. 1.
The effects of the stressor on a cortisol, b mean arterial pressure, and c subjective distress. For each outcome, the stressor group × time effect was significant. Bars indicate standard error. Significant timepoints where Stressor > no-stress control are indicated by asterisks (p<0.05). BL baseline, PT post-test measurement. Values in parentheses reflect min prior to start of the stressor (for BL) and min relative to the end of the stressor for PT. The increase in mean arterial pressure from PT1 to PT2 in the no-stress group coincided with delivery of the priming drink
Effect of the priming drink
The initial dose of alcohol was delivered to achieve a target BAC of 0.03 g/dl. The resulting BAC (assessed 15 min following consumption) did not differ significantly between stressor groups or genders, nor was there a gender × stressor group interaction. The overall mean for all participants was 0.029 g/dl (SE=0.001), which did not differ significantly from the 0.03 g/dl target. There was a trend for men to have higher BAC values than women, F(1,75)=3.39, p=0.07, and for control participants to have higher BAC values than the stressed group, F(1,75)=2.99, p=0.09. Because of these effects, BAC was examined as a covariate in subsequent analyses of the data from the taste test.
The initial dose of alcohol was expected to induce alcohol craving (as indexed by an increase in AUQ scores from 5:15 to 5:30), but this effect was not observed, F (1.75)<1.0, ns. There were no main or interaction effects with stressor group or gender on pre- vs. post-prime AUQ scores.
Stress-induced drinking
There was a trend for a positive correlation between drinks per day and amount of beer consumed in the taste test (r=0.21 p=0.07) and a significant correlation between drinks per day and whether the participant drank all the beer provided (Spearman’s rho=0.22, p<0.05). Drinks per day was examined as a covariate in these models, along with BAC and the covariates identified from assessment of psychological variables. Ultimately, none of these covariates accounted for variance in the dependent variables in such a way to alter the findings; unadjusted means are reported.
Amount of water consumed (milliliters) during the taste test was analyzed to examine effects of stress on nonspecific thirst. There was no effect of stressor group, gender, nor a stressor group × gender interaction on water consumed. On average, participants consumed 80 ml of water (SE=8.5) during the taste task.
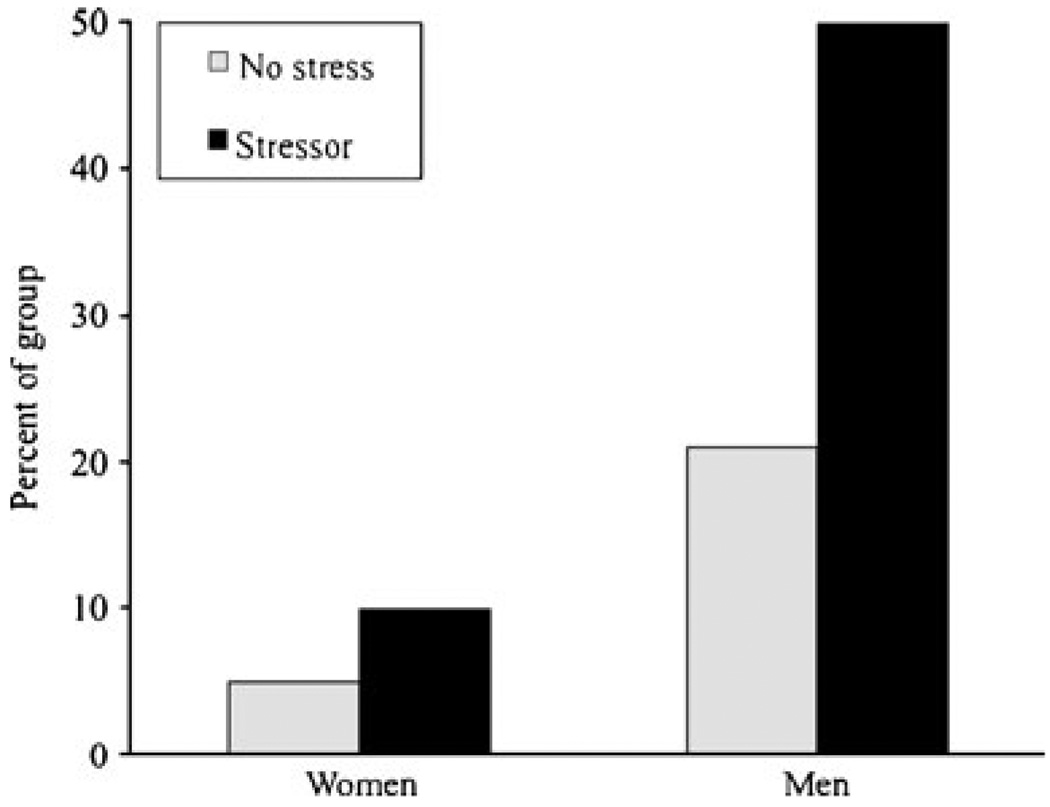
There was a main effect of the stressor on the probability of drinking all the beer available, Wald X2 (df, 1)=5.25, p<0.05, where exposure to the TSST doubled the probability of consuming all the beer (Fig. 2). There was not a stressor group × gender interaction on this outcome. There was no significant effect of stressor group nor a stressor group × gender interaction on milliliters beer consumed, F′s (1.74)<1.3, p ns.
Fig. 2.
Percent of each gender group that consumed all the beer available during the taste test. Stressor > no-stress (p<0.05) and men > women (p<0.05). There was no interaction between stress group and gender
There were main effects of gender on beer consumption. Men drank more milliliters of beer (M=542 ml, SE=28.4) than women (M=405 ml, SE=27.5), F(1,74)=11.34, p<0.01, and more men (36%) than women (8%) drank all the beer provided, Wald X2(df, 1)=8.29, p<0.01. Because genders were equally distributed in the stress and no-stress groups, main effects of gender did not directly impact power to reveal main effects of stress on these outcomes, although ceiling effects may have precluded our ability to detect gender × stress interactions on consumption. That is, though not statistically different, stressed men drank more beer than non-stressed men (577 vs 506 ml, both SEs~40); stressed and non-stressed women both drank about 405 ml (both SEs~40).
Secondary drinking measures (latency to first sip, median latency between sips, and sip size) were collected to examine the effect of the stressor on process measures. There was not a main effect of the stressor, or a stress × gender interaction on any of these outcomes. The only significant effect observed was on sip size, where men took larger sips than women (34 vs. 23 ml per sip, F(1,74)= 17.15, p<0.001). In addition, regardless of stressor group and gender, there was a significant positive correlation between sip size and drinks per day (r=0.26, p<0.05).
Alcohol craving during the challenge
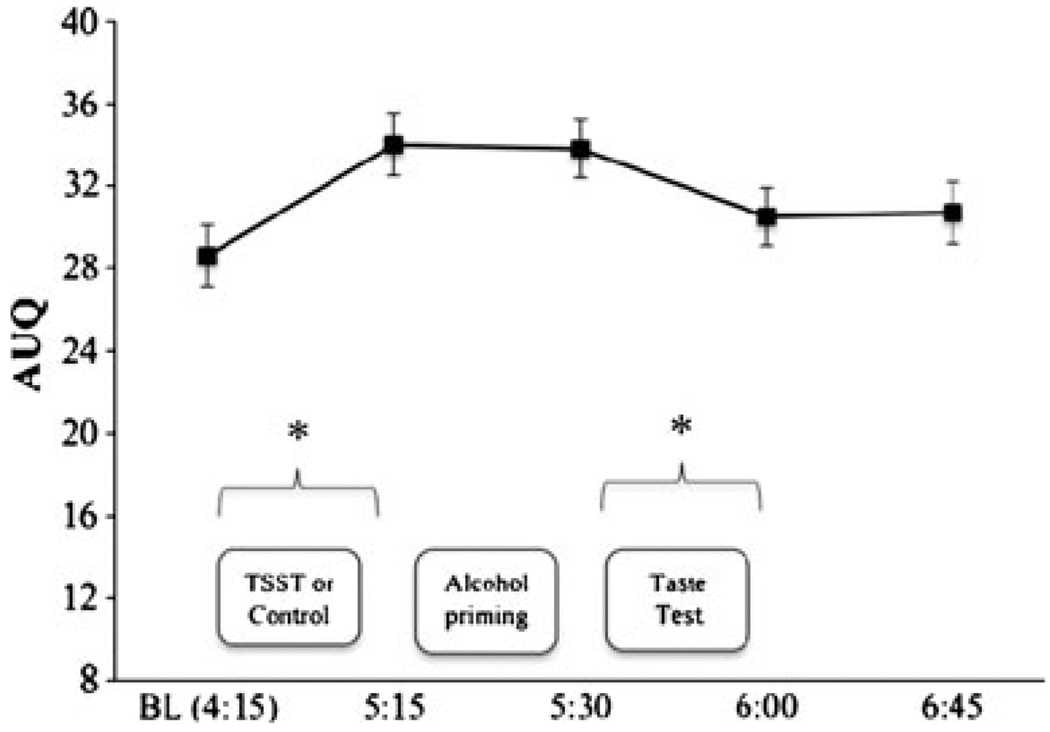
Covarying baseline AUQ scores and examining all time-points in a single model, there was no time × stressor group effect, nor a three-way interaction with gender, both p values>0.10. There was an effect of time [F(4,77)=13.51, p<0.01], as shown in Fig. 3 (bars show SE). AUQ scores increased from baseline to the post-stress assessment [t(78)=7.14, p<0.01], though not differently by stressor group. AUQ scores remained stable from pre- to post-alcohol priming drink (an unexpected finding, as reported above); AUQ scores significantly decreased pre- to post-taste test [t(78)=3.79, p<0.01] and remained stable from post-taste test to dismissal.
Fig. 3.
Mean (bars show SE) Alcohol Urge Questionnaire scores over the course of the challenge day collapsed across stressor groups. Asterisks reflect significant change over time (p<0.05). Craving increased from pre- to post-stress manipulation (but not differentially by stressor group). Alcohol craving decreased following the taste test (where participants were allowed to drink up to 24 oz of beer)
Discussion
The results of this clinical laboratory study demonstrate that an acute psychosocial stressor, the TSST, can promote drinking in both men and women with alcohol dependence, as individuals exposed to the stressor were twice as likely as non-stressed controls to drink the maximum amount available. Because the relationship between stress and drinking in alcoholics has not been widely examined using controlled experimental studies, and only one such study has included women (Pratt and Davidson 2009), the present study contributes valuable information to the extant literature and offers some guidance by its findings, both expected and unexpected, about how better to study this issue in the future.
While the stressed group was more likely to consume all the beer provided, there was no difference between groups on milliliters beer consumed. Ceiling effects may have limited our ability to detect this effect, especially for men, thus precluding our ability to observe a stress × gender interaction on alcohol consumption. We limited the available alcohol to 710 ml beer (24 oz, half from non-alcoholic beer) to minimize each participant’s peak blood alcohol level (both for safety and to reduce the time required for participants to remain in the laboratory following the challenge). As only 15 min were allotted for the taste test, we expected that this amount would be sufficient to test the primary hypotheses. To examine whether acute stress differentially affects drinking in men and women, future studies might increase the amount of alcohol offered and/or allow individuals to drink his/her preferred alcoholic beverage in the taste task. However, because there were no differences between gender or stress groups on the percent who preferred beer, it is unlikely that employing beer in the taste test accounted for the lack of a stress group × gender interaction on milliliters beer consumed.
With few exceptions, all experimental manipulations of the model worked as expected. The internal validity of the stress induction was supported by all measures of stress reactivity (cortisol, mean arterial pressure, and both instruments used to pressure, and subjective distress), and the amount of alcohol delivered in the priming dose achieved the target breath alcohol level. In addition, average number of drinks per day in the 30 days prior to consent was positively correlated with voluntary drinking in the taste test, thus supporting the ecological validity of the taste test. Although craving was not affected by the priming drink (in contrast to Davidson et al. 2003), craving was diminished when participants were permitted to drink all the beer available to them. Taken together, results suggest that the experimental procedures of the present study offered an effective balance between internal validity and ecological relevance.
Craving was unaffected by stressor, which contributes to a mixed body of literature on whether stress induces craving in alcoholics (Brady et al. 2006; Cooney et al. 1997; Fox et al. 2007; George et al. 2008; Mason et al. 2008; Pratt and Davidson 2009; Sinha et al. 2009; Thomas et al. 2011). Though not reported in the results, among stressed participants, there was no correlation between magnitude of change in craving following the TSST and amount of beer consumed or probability of drinking all the beer available. The disassociation between craving and consumption has been documented by others (Drummond et al. 2000; MacKillop et al. 2010), and Tiffany’s cognitive processing model of craving (1990) posits why craving may not lead to drinking. That model proposes that craving is a conscious process that is activated when drinking is prevented, either because the individual is attempting abstinence or alcohol is unavailable. In the present study, participants were not attempting abstinence, and all were aware that they would be drinking alcohol in the challenge, so neither of the situations that might induce craving was present.
Tiffany’s model may also be relevant in explaining the effects of stress on drinking. The model posits that for alcoholics, drinking is an over-learned and automatic action which requires conscious effort and cognitive resources to counter. Recent clinical laboratory studies show that a stressor can affect this balance in favor of the automatic behavior. Schwabe and Wolf (2010) recruited normal volunteers and reduced the hedonic value of a reward for which participants were trained to respond. Afterwards, they exposed some participants to an acute psychosocial stressor. During a subsequent extinction test, non-stressed participants reduced their responding to the devalued goal, whereas stressed participants continued to respond for the goal as they had before it was devalued, suggesting habitual learning was driving behavior. In a separate study in which pharmacological stressors were used, a similar result was found (Schwabe et al. 2010). In a study with male problem drinkers, a stressor increased a pre-existing bias to alcohol vs. neutral words (Zack et al. 2010). The results of these studies suggest that stress either promotes habitual responses, impedes ability to employ conscious processes to counter responding based on habit, or both. In terms of the cognitive processing model as it relates to participants in the present study, stress increased the likelihood of engaging in an automatized behavior, which, for this group of non-abstinent alcoholics, was to drink all the alcohol available.
Among the limitations of the study is the fact that selection factors reduce generalizability both to the behavior of interest (relapse drinking) and the population of interest (treatment-seeking alcoholics). For ethical reasons, only non-treatment-seeking alcoholics who were not attempting abstinence were included, so it is unknown whether these results apply as a model of stress-potentiated relapse. In addition, individuals were excluded from participating if they had comorbid psychiatric disorders and/or other drug dependence. As these conditions are common in alcoholics, especially in women and treatment-seeking samples (Kessler et al. 2005; Zilberman et al. 2003), results may not generalize to clinical populations. Another limitation is that the requested 48-h abstinence from alcohol prior to the challenge was confirmed only through self-report and negative breath alcohol reading. Since participants did not know to which group they were randomized prior to the challenge day, it is unlikely that stressor groups differed in adherence to the abstinence request, but we cannot confirm this assumption.
In summary, results suggest a possible causal relationship between stress and drinking in alcoholics. In addition, the methods may provide a foundation for future clinical laboratory studies on stress-induced drinking. Other clinical models of precipitants to relapse, including cue-induced craving and cue- and prime-induced drinking have been valuable in exploring individual differences in response to treatment and possible mechanisms of actions (Anton et al. 2004; Davidson et al. 2003; O’Malley et al. 2002; Sinha et al. 2009). A model of stress-induced drinking offers similar opportunities and could be used to examine whether interventions that have been shown to reduce stress reactivity may also reduce stress-potentiated or stress-induced drinking.
Acknowledgments
This research was supported by a grant from NIAAA to the Charleston Alcohol Research Center (P50 AA010761) and by support from the National Center for Research Resources (M01 RR01070).
Footnotes
Disclosures/conflict of interest The authors declare no conflicts of interest.
References
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacol Berl. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer R, Brown G. In: Manual for the Beck Depression Inventory-II. Corp P, editor. San Antonio, TX: 1996. [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall PK. Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol Clin Exp Res. 2006;30:938–946. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Caudill BD, Marlatt GA. Modeling influences in social drinking: an experimental analogue. J Consult Clin Psychol. 1975;43:405–415. doi: 10.1037/h0076689. [DOI] [PubMed] [Google Scholar]
- Conger JJ. The effects of alcohol on conflict behavior in the albino rat. Q J Stud Alcohol. 1951;12:1–29. [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, Skinner JB, Windle M. Development and validation of a three-dimensional measure of drinking motives. Psychol Assess. 1992;4:123–132. [Google Scholar]
- Davidson D, Tiffany S, Johnston W, Flury L, Li T. Using the cue-availability paradigm to assess cue reactivity. Alcohol Clin Exp Res. 2003;27:1251–1256. doi: 10.1097/01.ALC.0000080666.89573.73. [DOI] [PubMed] [Google Scholar]
- de Wit H, Soderpalm AH, Nikolayev L, Young E. Effects of acute social stress on alcohol consumption in healthy subjects. Alcohol Clin Exp Res. 2003;27:1270–1277. doi: 10.1097/01.ALC.0000081617.37539.D6. [DOI] [PubMed] [Google Scholar]
- Dickerson S, Kemeny M. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Phillips TS. Alcohol urges in alcohol-dependent drinkers: further validation of the Alcohol Urge Questionnaire in an untreated community clinical population. Addiction. 2002;97:1465–1472. doi: 10.1046/j.1360-0443.2002.00252.x. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Litten RZ, Lowman C, Hunt W. Craving research: future directions. Addiction. 2000;95:247–255. doi: 10.1080/09652140050111816. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong K-I, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Gronwall D. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Higgins RL, Marlatt GA. Effects of anxiety arousal on the consumption of alcohol by alcoholics and social drinkers. J Consult Clin Psychol. 1973;41:426–433. doi: 10.1037/h0035366. [DOI] [PubMed] [Google Scholar]
- Ice GH, James GD. Conducting a field study of stress: general principles. In: Ice G, James GD, editors. Measuring stress in humans: a practical guide for the field. New York: Cambridge University Press; 2007. p. 13. [Google Scholar]
- Kessler R, Chiu W, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Ten years of research with the Trier Social Stress Test—Revisited. In: Harmon-Jones E, Winkielman P, editors. Social neuroscience: integrating biological and psychological explanations of social behavior. New York: Guilford; 2007. pp. 56–83. [Google Scholar]
- Levy MS. Listening to our clients: the prevention of relapse. J Psychoactive Drugs. 2008;40:167–172. doi: 10.1080/02791072.2008.10400627. [DOI] [PubMed] [Google Scholar]
- MacKillop J. Factor structure of the alcohol urge questionnaire under neutral conditions and during a cue-elicited urge state. Alcohol Clin Exp Res. 2006;30:1315–1321. doi: 10.1111/j.1530-0277.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Miranda R, Monti P, Ray L, Murphy J, Rohsenow D, McGeary J, Swift R, Tidey J, Gwaltney C. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. J Abnorm Psychol. 2010;119:106–114. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse prevention: maintenance strategies in the treatment of addictive behaviors. New York: Guilford; 1985. [Google Scholar]
- Mason BJ, Light JM, Escher T, Drobes DJ. Effect of positive and negative affective stimuli and beverage cues on measures of craving in non treatment-seeking alcoholics. Psychopharmacol Berl. 2008;200:141–150. doi: 10.1007/s00213-008-1192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PM, Hersen M, Eisler RM, Hilsman G. Effects of social stress on operant drinking of alcoholics and social drinkers. Behav Res Ther. 1974;12:67–72. doi: 10.1016/0005-7967(74)90094-1. [DOI] [PubMed] [Google Scholar]
- Monti PM, Kadden RM, Rohsenow DJ, Cooney NL, Abrams DB. Treating alcohol dependence: a coping skills training guide. 2nd edn. New York: Guilford; 2002. [Google Scholar]
- National Advisory Council on Alcohol Abuse and Alcoholism. Recommended council guidelines on ethyl alcohol administration in human experimentation. [Accessed on: 1 Jan 2010];2005 doi: 10.1111/j.1530-0277.2009.00988.x. Available at http://www.niaaa.nih.gov/Resources/ResearchResources/job22.htm. [DOI] [PMC free article] [PubMed]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamopituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pratt WM, Davidson D. Role of the HPA axis and the A118G polymorphism of the mu-opioid receptor in stress-induced drinking behavior. Alcohol Alcohol. 2009;44:358–365. doi: 10.1093/alcalc/agp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Sayette MA. An appraisal-disruption model of alcohol’s effects on stress responses in social drinkers. Psychol Bull. 1993;114:459–476. doi: 10.1037/0033-2909.114.3.459. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf O. Socially evaluated cold pressor stress after instrumental learning favors habits over goal-directed action. Psychoneuroendocrinology. 2010;35:977–986. doi: 10.1016/j.psyneuen.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Tegenthoff M, Hoffken O, Wolf O. Concurrent glucocorticoid and noradrenergic activity shifts instrumental behavior form goal-directed to habitual control. J Neurosci. 2010;30:8190–8196. doi: 10.1523/JNEUROSCI.0734-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Sher KJ, Levenson RW. Risk for alcoholism and individual differences in the stress-response-dampening effect of alcohol. J Abnorm Psychol. 1982;91:350–367. doi: 10.1037//0021-843x.91.5.350. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Litten RZ, Allen JP. Timeline follow-back: a technique for assessing self-reported alcohol consumption measuring alcohol consumption: psychosocial and biochemical methods. Totowa: Humana; 1992. pp. 41–72. [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto: Consulting Psychologists; 1983. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Randall PK, Brady KT, See RE, Drobes DJ. An acute psychosocial stressor does not potentiate alcohol cue reactivity in non-treatment seeking alcoholics. Alcohol Clin Exp Res. 2011;35 doi: 10.1111/j.1530-0277.2010.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Vieten C, Astin JA, Buscemi R, Galloway GP. Development of an acceptance-based coping intervention for alcohol dependence relapse prevention. Subst Abus. 2010;31:108–116. doi: 10.1080/08897071003641594. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Tucker JA. Alcoholic relapse, life events, and behavioral theories of choice: a prospective analysis. Exp Clin Psychopharmacol. 1996;4:19–28. [Google Scholar]
- Watson P. Total body water and blood alcohol levels: updating the fundamentals. In: Crow K, Blatt R, editors. Human metabolism of alcohol. Boca Raton: CRC; 1989. pp. 41–56. [Google Scholar]
- Zack M, Woodford TM, Tremblay AM, Steinberg L, Zawertailo LA, Busto UE. Stress and alcohol cues exert conjoint effects on go and stop signal responding in male problem drinkers. Neuropsychopharm. 2010 doi: 10.1038/npp.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman M, Tavares H, Blume S, el-Guebaly N. Substance use disorders: sex differences and psychiatric comorbidities. Can J Psychiatry. 2003;48:5–13. doi: 10.1177/070674370304800103. [DOI] [PubMed] [Google Scholar]
- Zywiak WH, Connors GJ, Maisto SA, Westerberg VS. Relapse research and the reasons for drinking questionnaire: a factor analysis of Marlatt’s relapse taxonomy. Addiction. 1996;91 Suppl:S121–S130. [PubMed] [Google Scholar]