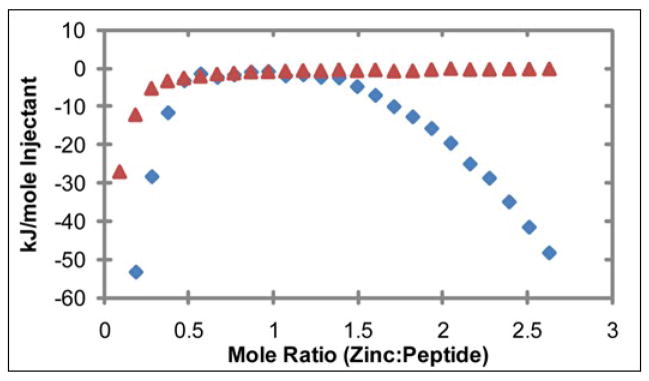
Figure 4.
Heat evolved from the titration of ZnCl2 into hIAPP1–19 at pH 7.3 and 25°C, as measured by ITC. In solution with 100 mM Tris buffer and 100 μM NaCl (
 ), zinc has one apparent binding site at sub- stoichiometric ratios of 0.136 zinc to 1 hIAPP1–19. In the presence of 30% TFE (
), zinc has one apparent binding site at sub- stoichiometric ratios of 0.136 zinc to 1 hIAPP1–19. In the presence of 30% TFE (
 ) the peptide adopts an α-helical conformation, causing a shift in the apparent binding stoichiometry of the primary binding site to 0.24 zinc to 1 hIAPP1–19 and the addition of an endothermic process at higher concentrations of zinc.
) the peptide adopts an α-helical conformation, causing a shift in the apparent binding stoichiometry of the primary binding site to 0.24 zinc to 1 hIAPP1–19 and the addition of an endothermic process at higher concentrations of zinc.