Table 3.
IC50 values and selectivity ratios for substituted A-ring analogues.
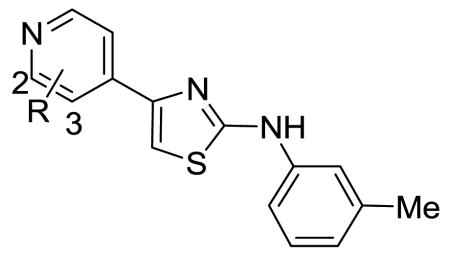 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | 3-R | RCC4 IC50 μM | RCC4/VHL IC50 μM | Ratioa | No | 2-R | RCC4 IC50 μM | RCC4/VHL IC50 μM | Ratioa |
| 18 | Ph | 11.6 | 33.2 | 2.9 | 39 | 2-Ph | 19.1 | 40.0 | 2.1 |
| 19 | 4-pyridyl | 13.8 | 34.0 | 2.5 | 40 | Ph-4-Ac | 38.8 | 33.4 | 0.9 |
| 20 | Ph-4-CF3 | 12.8 | 22.6 | 1.8 | 41 | 4-pyridyl | 28.9 | 38.0 | 1.3 |
| 21 | Ph-4-iPr | >40 | >40 | ND | 42 | 2-pyridyl-6-F | >40 | >40 | ND |
| 22 | Ph-4-CONH2 | 4.1 | 1.6 | 1.6 | 43 | 5-pyrimidine | 26.4 | 37.0 | 1.4 |
| 23 | Ph-4-CONpiperazine-N-methyl | 12.8 | >40 | >3.1 | 44 | 4-(1H-indole) | 3.1 | 4.5 | 1.5 |
| 24 | Ph-4-CONH(CH2)3NMe2 | 4.0 | 3.9 | 1.0 | 45 | 3-thiophene | 8.7 | >40 | >4.6 |
| 25 | Ph-4-SO2CH3 | 1.6 | 13.4 | 8.4 | 46 | 4-(3,5-dimethyloxazole) | 3 | 36.5 | 12 |
| 26 | Ph-4-SO2NHtBu | 4.5 | 22.5 | 5.0 | 47 | 3-pyrazole | 0.48 | 20.0 | 14 |
| 27b | Ph-4-Ac | 3.9 | 35.1 | 9 | 48 | 4-(1-methylpyrazole) | 5.2 | 8.1 | 1.6 |
| 28b | C≡CCH2OH | 0.24 | 2.9 | 11.9 | 49 | 4-(1-benzylpyrazole) | 2.2 | 66.1 | 30 |
| 29b | C≡CCH2OMe | 0.62 | 27.9 | 45 | 50 | 4-(1-benzyltriazole) | 2.1 | 14.0 | 6.7 |
| 30 | C≡C(CH2)2OH | 1.1 | 2.8 | 2.5 | 51 | 4-methylpiperazine | 11.5 | 31.6 | 2.8 |
| 31 | C≡C(CH2)3OH | 6.3 | 0.3 | 0.1 | 52 | NH(CH2)2Nmorpholine | 3.7 | 8.0 | 2.2 |
| 32 | C≡CCH2Nmorpholine | 0.57 | 2.3 | 4.0 | 53 | O(CH2)2Nmorpholine | 7.0 | 21.1 | 3.0 |
| 33 | C≡CCH2Npiperazine-4-Me | 2.6 | 2.2 | 0.9 | 54 | O(CH2)3NMe2 | 6.0 | 8.8 | 1.5 |
| 34 | 4-(1-benzyltriazole) | >40 | >40 | ND | |||||
| 35 | 4-methylpiperazine | 2.6 | 6.6 | 2.5 | |||||
| 36 | NH(CH2)2Nmorpholine | 19.5 | 27.9 | 1.4 | |||||
| 37 | O(CH2)2Nmorpholine | 0.32 | 0.88 | 2.8 | |||||
| 38 | O(CH2)3NMe2 | 2.7 | 20.2 | 7.4 | |||||
Ratio = IC50 (RCC4/VHL)/IC50 (RCC4).
Data from reference 20.
