Abstract
Heart failure (HF) is a modern epidemic and is one of the few cardiovascular diseases which is increasing in prevalence. The growing importance of the Natriuretic Peptide (NP) system in HF is well recognized. Laboratory tests for B-type Natriuretic Peptide (BNP) have proven value as diagnostic and prognostic tools in HF and are now part of routine clinical care. Furthermore, recombinant atrial natriuretic peptide (ANP) (carperitide) and BNP (nesiritide) and are approved HF therapies in Japan and the US, respectively and additional natriuretic peptides (e.g., CNP, urodilatin, and designer NPs) are under investigation for use in HF. Common genetic sequence variants are increasingly being recognized as determinants of disease risk or drug response and may help explain a portion of the inter-individual variation in the human NP system. This review describes current knowledge of NP system genetic variation as it pertains to HF as well as ongoing studies and where the field is expected to progress in the near future. To briefly summarize, NP system genetic variants have been associated with alterations in gene expression, NP levels, and cardiovascular disease. The next step forward will include specific investigations into how this genetic variation can advance ‘Personalized Medicine’, such as whether they impact the utility of diagnostic BNP testing or effectiveness of therapeutic NP infusion. This is already in progress, with pharmacogenetic studies of nesiritide currently underway. We expect that within 5 years there should be a reasonable idea of whether NP system genetic variation will have important clinical implications.
Keywords: BNP, Heart failure, Pharmacogenetics
Introduction
Heart failure (HF) is a modern epidemic and is one of few cardiovascular diseases which is increasing in prevalence [1, 2]. At a time when mortality from coronary artery disease and stroke is declining, HF is a growing public health problem. According to the American Heart Association, HF afflicts over 500,000 Americans annually, and the prevalence of HF approaches 5 million cases [2]. It remains a highly lethal and costly disease [1], with a one year mortality of 45% [3]. Acutely exacerbated HF in particular is a considerable public health issue. According to the National Center for Health Statistics, in-patient treatment for HF accounted for 5.3 million hospital days in 1999 and is the second most frequent diagnosis for patients ages 65 and older [4].
The importance of the Natriuretic Peptide (NP) system in cardiovascular homeostasis and HF has been increasingly well recognized since the recognition of the existence of an atrial natriuretic factor in the late 1970s. Since then, the individual peptide hormones have been isolated, their receptors defined, and their functional importance delineated through many experiments in both humans and animals including very elegant knockout mouse models. This has led to our current understanding of the NP system, as an important counter-regulatory system that reduces blood pressure (BP) and has generally salutary effects in the HF milieu through a myriad of mechanisms that include direct vasodilitation, natriuresis, vascular permeability, and indirect effects on other regulatory pathways, and others.
This vital physiologic importance is now also reflected in the widespread clinical applications involving the NP system. All three naturally occuring NPs and some of related proteins have been the subject of intense study and are at various stages of being incorporated into clinical medicine. The most developed example is B-type Natriuretic Peptide (BNP). Laboratory tests for BNP and N-terminal-proBNP have proven value as a diagnostic and prognostic tools in HF and are now part of routine clinical care [3, 5, 6]. Furthermore, recombinant BNP, known as nesiritide, is an approved therapy for acutely decompensated HF [7]. The other NPs are also the subject of investigation and are likely to have clinical and scientific implications. In fact, recombinant atrial natriuretic peptide (ANP) known as carperitide is used to treat HF in Japan [8], and other NPs such as C-type natriuretic peptide (CNP), and the renal-tubular isoform of ANP (urodilatin), and other ‘designer’ peptides are under active investigation for use in HF.
Common genetic sequence variants are increasingly being recognized as determinants of disease risk or drug response in general, [9–11] and may help explain a portion of the inter-individual variation in the human NP system in particular. Indeed, substantial genetic variation in NP system components have been documented (Table 1), some of which have been associated with altered biologic function and disease [12]. These and other variants may also have further clinical implications in terms of diagnostic, prognostic, and therapeutic uses. This review will briefly summarize NP system biology, describe the current knowledge of NP system genetic variation as it pertains to HF diagnosis and treatment, and finally address ongoing studies and where the field is expected to progress in the near future.
Table 1.
NP system gene names, and summary information
| Gene | HUGO symbol | Aliases | Length (kb) | Exons | #SNPs* |
|
|---|---|---|---|---|---|---|
| Total | Coding | |||||
| Natriuretic peptide precursor A | NPPA | 2 | 3 | 35 | 5 | |
| Natriuretic peptide precursor B | NPPB | 1.5 | 3 | 27 | 7 | |
| Natriuretic peptide precursor C | NPPC | 1 | 2 | 16 | 4 | |
| Serine peptidase | CORIN | Corin, ATC2, CRN, Lrp4, heart specific serine proteinase, pro-ANP convertase | 240 | 22 | 879 | 11 |
| Natriuretic peptide receptor 1 | NPR1 | NPR-A, GC-A | 16 | 22 | 69 | 16 |
| Natriuretic peptide receptor 2 | NPR2 | NPR-B, GC-B | 17 | 22 | 69 | 14 |
| Natriuretic peptide receptor 3 | NPR3 | NPR-C | 75 | 8 | 326 | 4 |
| Membrane metallo-endopeptidase | MME | Neprilysn, neutral endopeptidase, NEP | 100 | 24 | 360 | 12 |
From dbSNP (http://www.ncbi.nlm.nih.gov/sites/entrez) 1/4/2008
NP system overview
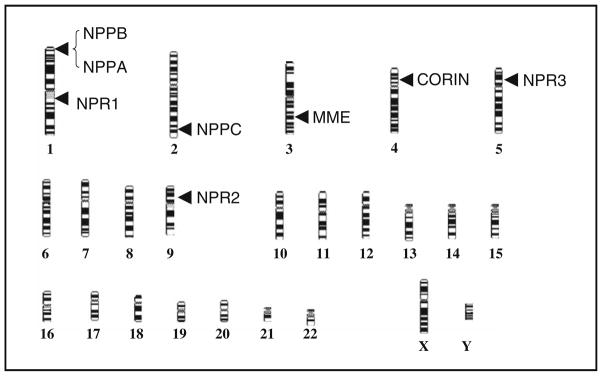
The NP system that will be discussed below can be thought of as incorporating NP production, the NP receptors (NPR), and NP clearance mechanisms (Fig. 1). The genes encoding NP system components, their HUGO symbols, lengths, number of exons, and chromosomal locations are summarized in Table 1 and Fig. 2.
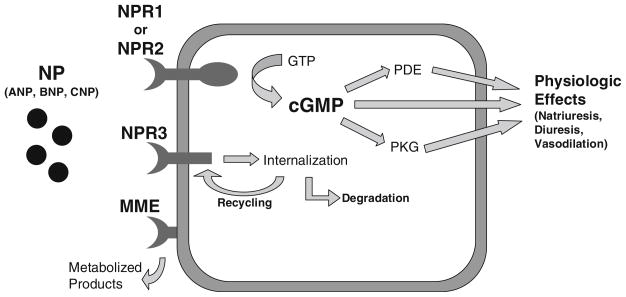
Fig. 1.
BNP pathway showing key effector and clearance mechanisms, Natriuretic Peptide Receptor 1 (NPR1), NPR2, NPR3, and Membrane Metallo-Endopeptidase (MME) (adapted from Stoupakis et. al [14])
Fig. 2.
Chromosomal locations of NP system genes
Atrial natriuretic peptide, BNP, and CNP are produced as precursor peptides by the genes natriuretic peptide precursor-A (NPPA), NPPB, and NPPC, respectively. The precursors are processed and ultimately cleaved into the active forms of the peptides by serine peptidase (a.k.a. corin, pro-ANP converting enzyme), produced by the gene CORIN. Each peptide is produced mainly in the heart (though there are much lesser amounts in certain others tissues). ANP has relatively greater atrial production compared to BNP, which is predominantly produced in the ventricular myocardium. ANP is also generated in the kidney where tissue specific, alternative processing produces urodilatin. BNPs production is even more predominantly of ventricular origin in the setting of HF where there is often increased ventricular mass and filling pressures resulting in the vast majority of BNP being generated there. NPPB gene regulation is complex and has been described in full detail elsewhere [13], but a key component is myocardial filling pressures which effect transcription via stretch response elements in the NPPB promoter. All the NPs have a disulfide bond with a shared ring structure that is important in their binding to the NPRs.
The NP system components responsible for imparting biologic effects are the NPR 1 and 2 (a.k.a. NPRA and NPRB, GC-A and GC-B). These are both membrane bound guanylate cyclases. Specific binding of NPs to NPR1 or NPR2 initiates enzymatic activity, increasing production of cyclic guanosine monophosphate (cGMP). CGMP is the second messenger which mediates NP biologic effects. These include natriuresis, vasodilation, and others. The NPs can display differing physiology based on their distinct affinities (Table 2) for NPR1 versus NPR2 resulting differences in the relative amount of vasodilation, natriuresis, etc. that they cause [14, 15]. Both NPR1 and NPR2 are present in many tissue types including the vasculature (endothelium and smooth muscle), heart, and kidney. They are produced by similarly named genes NPR1 and NPR2.
Table 2.
Dissociation constants for combinations of NP to receptor binding
| Peptide | Receptor
|
||
|---|---|---|---|
| NPR1 | NPR2 | NPR3 | |
| ANP | 1.9 pM | 5,400 pM | 2.6 pM |
| BNP | 7.3 pM | 30,000 pM | 13 pM |
| CNP | >500,000 pM | 7 pM | 10.8 pM |
Taken from Koller et al. [15]
Natriuretic Peptides are cleared by two primary mechanisms. First, is NPR3 which is a non-catalytic receptor which leads to peptide internalization and lysosomal degradation [16, 17]. It shares homology with NPR1 and 2 in the extra-cellular portion but lacks the guanylate cyclase domain. The second clearance mechanism is through enzymatic cleavage by Membrane Metallo-Endopeptidase (MME, a.k.a., Neutral Endopeptidase) [16]. There is also a smaller component of renal filtration. Both NPR3 and MME are expressed in multiple tissues including lungs and kidney. Current data suggests that these two are also the key clearance mechanisms for exogenously delivered NP (e.g., nesiritide, carperitide) even though higher NP levels are observed with pharmacologic infusion [7, 16].
As indicated above, the NP system has an important role in hemodynamic regulation, acting through a number of mechanisms both direct and indirect. Most of these actions affect vascular volume and tone resulting in lowering of BP. In terms of the direct volume regulation, this seems to be accomplished through multiple pathways. Natriuresis is certainly a contributor, [18] though its extent remains controversial, and an important component of vascular permeability regulation has been recognized [19]. Direct effects on vascular tone are more straightforward with the system having well-recognized direct vasodilating properties, particular via NPR1 [20].
In addition to these direct hemodynamic properties, the NP system also has many other effects. The system has indirect hemodynamic effects via antagonization of the Renin-Angiotensin-Aldosterone system and the adrenergic system which also contribute to lowering of BP and vascular tone. The NP system also shows anti-fibrotic effects [21, 22], and inhibition of NF-kappaB [23] and TGF-β [24]. Along these lines, it is interesting that the predominant phenotype of NPPA and NPR1 knockout mice is arterial hypertension and myocardial hypertrophy [25, 26], while NPPB knockouts displayed a tendency towards cardiac fibrosis without hypertension [27]. Underscoring the still developing and incomplete nature of our understanding of the NP system is recent data pointing to NPR3 coupling to inhibitory G-proteins causing inhibition of adenyl cyclase A, phospholipase-C as well as inhibitory effects on L-type calcium channels [17].
Evidence of altered biologic function and disease risk based on genetic variation in the NP system
Genetic variation within the NP system is well documented. The NCBI polymorphism database (DbSNP) shows nearly 2,000 documented variants in NP system genes (Table 1) of which 73 are coding variants, and there are additional length variants and deletions not included. Many research groups including our own have described common polymorphisms and haplotype structure of the key genes in the NP system [28–32]. Blood BNP levels have been shown to be heritable, [33] supporting the notion that genetic variation in the system impacts its function. Moving beyond simple validation of the presence of polymorphisms, numerous sequence variants in NP system genes have been further scrutinized, with indications that many alter the function of the resulting protein. This evidence ranges from functional data such as gene expression, to association with cardiovascular phenotypes. [34–38] In terms of disease risk associations, NP system variants have been associated with several HF preconditions including hypertension (the most frequently noted), myocardial hypertrophy, as well as myocardial infarction and others.
Natriuretic peptide receptor 1
Natriuretic peptide receptor 1 is probably the NP system gene studied in greatest detail at present. There is a strong accumulation of evidence that NPR1 genotype impacts phenotype, spanning from animal models to humans and from molecular to clinical phenotypes. An interesting example at the mechanistic level is an elegant study by Knowles and colleagues, which demonstrated that sequence variants in NPR1 significantly impacted gene expression. This study examined ten non-coding variants (Five SNPs and five length-repeat polymorphisms) and tested their association with transcript levels using reporter plasmids in cultured cells. They found that 5′ flanking region haplotypes were associated with up to a 2-fold difference in reporter expression [34]. Interestingly, this parallels rat model data where a length-repeat variant in the promoter region correlated with gene expression as well as diastolic BPs [39]. Consistent with this line of evidence is a study in humans, which showed that a microsatellite polymorphism in the 5′ flanking region was associated with cardiac hypertrophy among hypertensive patients [40]. In this study the rare variant (which made up 9% of the population) had roughly 20% greater septal thickness and left ventricular mass index by echocardiogram compared to the other genotypes.
The theme of NPR1 sequence variants associating with hypertension is a recurrent one, in fact one of the first published associations was a deletion mutation in the NPR1 promoter which is 8 times more common among Japanese hypertension patients than those without hypertension [37]. This variant has a known biologic mechanism as well; the 8 bp deletion is in the 5′ flanking region (5′FR) of the gene, interrupts a key transcription factor binding site, and results in <30% transcriptional activity compared to the wild-type [37]. While quite compelling, this observation has not been replicated in other racial groups, so its relevance to disease in other populations remains to be proved. In fact, our group and others have failed to find this variant in Caucasians or African Americans [41, 42]. Additional variants in NPR1 have been associated with other cardiovascular phenotypes. A coding variant in exon 3 (1,023 GT) has been shown to be associated with myocardial infarction [35] and altered BNP:blood pressure ratio [43].
Other NP receptor genes
Variants in both NPR2 and NPR3 have been also associated with cardiovascular phenotypes. A string of experiments from Rahmutula and colleagues interrogating NPR2 revealed several polymorphisms and associations to HTN. They describe a dinucleotide repeat polymorphism in intron 2 (in strong linkage disequilibrium with a synonymous coding variant in exon 11 [31]) which was associated with HTN [44]. This variant carried a 1.55 odds ratio of HTN (P = 0.026). This again was performed in Japanese subjects and validation in other racial groups has not been performed. For NPR3, Sarzani and colleagues identified a SNP near the transcription start site within a conserved element. This promoter variant, −55 CA has been linked to a family history of HTN [36], altered ANP levels [45], and higher BP in obese hypertensive patients [46].
Other NP system candidate genes
Variants in the genes encoding the NPs themselves have also been linked to various phenotypes. ANP and BNP are well known having been described long ago, and thus their genes, NPPA and NPPB, have the most data regarding their sequence variants and phenotypic changes. In contrast, there is little published to date in terms of functional variants and cardiovascular phenotypes for MME, NPPC, or CORIN. MME is an interesting case as there are numerous studies examining sequence variants and the association with dementia or angiopathy since this enzyme is also thought to break down amyloid β protein, however these have yielded inconsistent results [47, 48].
In terms of NPPA, sequence variants have been associated with ANP level and clinical phenotypes such as HTN [49]. An example with multiple studies of the same variant is the T2238C variant. It has been associated with NP levels among HF patients [45], and has also shown a strong association with the risk of stroke [50], both in Italian populations. In this latter study, the odds ratio for stroke using multiple regression was 3.8 for CC versus TT genotypes (P = 0.04). While stroke is not directly related to HF, this association implies that the variant may have hypertensive or ischemic characteristics, which could lead to HF.
Natriuretic peptide precursor-B variants have also been evaluated with several interesting phenotypic correlations being documented. At the molecular level, the −381 CT variant was shown to impact gene expression [51]. The authors further showed this variant was associated with diabetes mellitus and validated the finding in multiple cohorts. In terms of cardiac phenotypes, a length polymorphism showed a weak association with HTN among Japanese females (P = 0.046) [52], but two studies have shown no association to cardiomyopathy. In one convincing study, there was no association between NPPB variants and hypertrophic cardiomyopathy when tested systematically via sequencing in 238 affected patients [53]. Another study looked for associations of NPPB to dilated cardiomyopathy and found none, but it is not definitive since only a single variant (C-1563T) was tested [54].
Genetic variants influencing BNP levels and cardiovascular homeostasis: potential implications for BNP testing
B-type natriuretic peptide levels have been well established as important diagnostic and prognostic markers in HF and other cardiovascular diseases [55–57]. Our group has previously demonstrated that BNP levels are strongly associated to left ventricular end-diastolic pressure (LVEDP) [58], as well as the risk of hospitalization among elderly cardiac patients [59]. However, the optimal interpretation of native serum BNP concentration remains controversial [57, 60]. Despite strong statistical relationship of BNP levels to cardiac filling pressures, the correlation is imperfect and does not allow for estimation of cardiac filling pressures non-invasively. Further, the biologic and physiologic factors that govern BNP levels and their interaction with cardiovascular parameters remain incompletely understood [60, 61]. Greater understanding of the genetic influences on NP system function may allow for improved utilization of BNP levels clinically.
To begin to dissect the impact of genetic variation on BNP levels and what they mean clinically, a first step is to investigate how candidate variants influence the relationship of BNP to important cardiovascular phenotypes. For example, as mentioned above, Nakayama and colleagues were able to show that an NPR1 variant was associated with higher BNP:BP ratio [43]. This might indicate that the variant for whatever reason had decreased function, requiring greater BNP levels to achieve hemodynamic homeostasis. It would then be reasonable to question whether carriers of such a variant would have higher baseline BNP levels, and whether the BNP value should be interpreted differently depending on genotype. In similar fashion our group investigated whether variants in NP system genes would influence the BNP-LVEDP relationship. LVEDP is a key determinant of cardiovascular status, with high LVEDP being a hallmark of HF. Our group and others have established an important relationship between BNP levels and LVEDP [56, 58, 62], which is mediated partially via stretch responsive elements in the natriuretic peptide precursor B (NPPB) promoter [63]. If genotype importantly affected the BNP-LVEDP relationship, this would certainly be expected to affect the thresholds of BNP used for diagnosis of acute HF, and perhaps even prognosis.
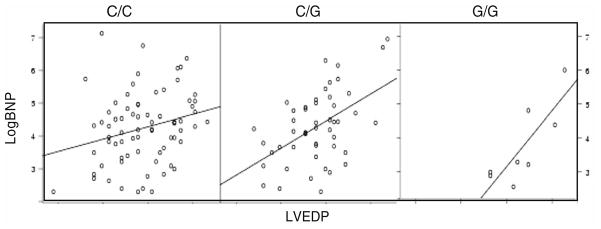
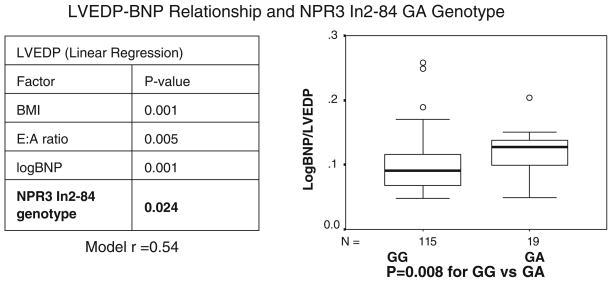
The association of genotype with logBNP:LVEDP ratio was tested in 147 patients undergoing routine cardiac catheterization (after excluding those with acute myocardial infarction). This revealed several interesting relationships between genotype BNP:LVEDP ratio for NPR2, NPR3, and NPPB. For example, the BNP:LVEDP ratio differed significantly by NPR2 In18–172 CG genotype (P = 0.02, unpublished data). As depicted in Fig. 3, the patients with NPR2 In18 −172 G had a steeper BNP:LVEDP slope, indicating progressively higher BNP levels for a given LVEDP, depending on the number of G alleles present. While a difference in function cannot be proven from this data, this pattern is consistent with relatively decreased NPR2 function in the G allele carriers. An even stronger relationship was discovered with an NPR3 variant. The NPR3 IVS-84 GA polymorphism showed a very strong relationship with LogBNP:LVEDP (Fig. 4, P = 0.008) [28]. The association of genotype to LVEDP was further analyzed in multiple regression models of LVEDP, in order to account for baseline covariates. In linear regression models of LVEDP genotype remained a significant predictor (P = 0.024) [28]. Other authors have found a bivariate association between an NPR3 5′ flanking variant and BNP levels among patients with HF [45].
Fig. 3.
LogBNP versus LVEDP scatterplot by NPR2 In18 −172 CG genotype
Fig. 4.
LVEDP-BNP relationship and NPR3 In2–84 GA genotype
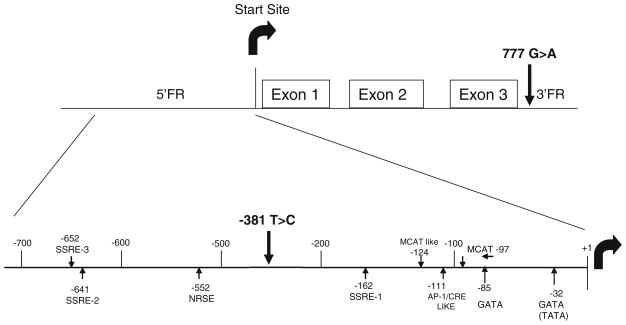
Another way to analyze the relationship of NP system sequence variants to cardiovascular status, which better account potential confounders, is to model BNP levels in multiple regression models. We took this approach with the dataset above, examining 19 variants in four NP system candidate genes, and testing the incremental predictive power of genotype for BNP after adjustment for baseline characteristics including LVEDP. This revealed associations of two promoter variants of NPPB with BNP levels. The strongest association was with the NPPB −381 TC variant (P = 0.0005), though another promoter variant −777 also showed a weaker association [41]. Interestingly, NPPB haplotype did not offer additional information over genotype alone. This finding was recently corroborated in another study, where the authors used a luciferase reporter gene in cultured cells and found a 1.8-fold increased expression for the −381 C allele promoter versus the T allele [51]. While no known binding site for a transcription factor relevant to NPPB is impacted by this variant, given its location (Fig. 5) it still seems likely that the effect is mediated via transcriptional regulation. These data indicate that NPPB promoter variants are a key source of inter-individual variation in BNP levels, and may impact the clinical interpretation of BNP testing. Certainly the magnitude of effect that we observed is well within the range of clinical significance; based on our linear regression model, a patient homozygous for the NPPB −381 T allele would be expected to have a BNP level approximately one half that of a similar patient homozygous for the C allele.
Fig. 5.
NPPB gene structure depicting regulatory sites, exons, start site, and variant loci (adapted from LaPointe et. al. [63])
In similar fashion an NPPA variant has been associated with ANP and BNP levels among HF patients [45]. This association withstood adjustment for baseline covariates (though this study did not include a measurement of filling pressures such as LVEDP). While the association of NPPA variants with ANP levels would seem straightforward (perhaps effecting gene function or regulation), the association with BNP levels is less so, perhaps suggesting a regulatory interaction or that the variant is associated with disease severity.
Future investigations need to focus on incorporating genotype into well-defined clinical cohorts that include BNP testing and can assess clinical outcomes among HF or coronary disease patients. To our knowledge there are no such studies published. The optimal use of BNP testing remains controversial and it is possible that genetic testing can lend some clarity to the wide variations between and within individuals, and therefore help define what BNP levels mean on an individual basis. An emerging area where this might also be relevant is with other BNP forms which may be measured. Most readers will be familiar with N-terminal pro-BNP (NTproBNP). More recently developed are tests for proBNP (the entire pro-peptide prior to cleavage, 108 amino acids). Which test is most clinically informative is a current research question under active investigation [64]. It would seem likely that genetic variants will play into this. For example, since CORIN activity is required to cleave proBNP into NTproBNP and BNP, variants associated with CORIN function would be expected to lead different levels in otherwise similar clinical circumstances. Determining which one best reflects the clinical situation may be dependent on the genetic milieu of the patient.
Pharmacogenetics of the NP system: rationale and ongoing studies
The line of evidence described thus far demonstrates that genetic variants in NP system genes are functional and associated with important phenotypic changes. Such functional differences could reasonably be expected impact the effect of exogenous NP infusion as well. For example, if a variant in NPR3 is associated with decreased clearance receptor function one would expect higher and perhaps toxic BNP levels to be achieved with standard infusion. Certainly therapeutic BNP infusion is a treatment modality in great need of optimal targeting. Nesiritide (Natrecor* by SCIOS), the commercially available recombinant BNP, is identical to the naturally occurring peptide and has salutary effects on symptoms and hemodynamics in HF patients [65, 66]. When first released, nesiritide was quickly integrated into the treatment of acutely decompensated HF. The rapid rise of its use was due to several factors. It was the first agent specifically aimed at and approved for decompensated HF in many years. With an amount of data that at the time dwarfed previous randomized trials in acutely decompensated HF, it is one of very few treatments that have been proven to be symptomatically beneficial to these patients. Further, it does not appear to elevate arrhythmic risk and has not been definitively shown to affect mortality (both are significant advantages over inotropic therapy) [65–67].
Despite these advantages nesiritide use does carry with it reasons to be cautious. Nesiritide infusion can cause significant hypotension, and this is in fact the most significant side effect of treatment. In addition, it is only available as an intravenous infusion, making it sometimes difficult to use. It is also quite expensive; each day a patient is treated represents over $600 in additional pharmacy cost. The possibility of other serious adverse effects is of concern as well. In 2004 great attention was focused on two meta-analyses, which showed possible links to worsening renal function [68], and increased mortality [69]. These were followed by a highly publicized editorial in the New England Journal of Medicine highlighting these results and the safety concerns. As a result nesiritide use sharply declined. These studies are not conclusive, but they were provocative correctly spurring more research into the benefits and risks of nesiritide therapy and how it should be used. The high cost, the risk of hypotension, and concern about renal failure underscore the need for investigations to identify more precisely which patients can benefit from nesiritide therapy, and in which patients it should be avoided due to excess risk or lack of efficacy. Nesiritide, like most pharmacologic treatments, remain imprecisely targeted. Certainly there would be great potential benefit if improved predictors of BNP response (both favorable and unfavorable) could be identified.
Fortunately, two ongoing studies should largely answer the above questions and concerns. These aim to address whether genetic determinants will predict patients’ response to infused BNP in terms of BNP clearance, cellular effects, physiologic effects, and clinical outcomes. The first is a small investigator initiated study by our group of collaborators which aims to quantify patients’ response to nesiritide. A total of 250 subjects are being recruited, who are planned to receive nesiritide therapy. Individual response to drug is being quantified in terms of BNP level achieved, BNP clearance, cGMP production, as well as hemodynamic responses. This study will be able to establish or refute physiologic differences in response according to common genotypes.
While this study could establish proof of principle and possible underlying mechanisms, it certainly is not adequately powered to assess clinical outcomes or the association of genotype with them. For that a definitive, multi-center, randomized clinical trial is underway. The Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) is currently enrolling and plans to recruit 7,000 subjects world wide. This is the largest study of ADHF ever undertaken. It is adequately powered to evaluate not only symptom improvement but combined death or rehospitalization at 30 days. This should give clinicians a much better idea of the efficacy and proper use of nesiritide in ADHF. Even more exciting is the fact that collection of genetic information was incorporated into the study from the beginning so that all subjects who choose to participate in the genetic portion will have their DNA collected and stored for analysis. In concert with the physiologic data that should be available from the first study described above, this should definitively establish whether genetic determinants can allow more efficient use of nesiritide. Unfortunately, the size of the study dictates that a long enrollment period is needed. Therefore no data can be expected from this landmark experiment for at least 4–5 year. However, since the primary endpoints are of short timeline (from hospitalization to 30 days) results should quickly follow completion of enrollment.
While recombinant BNP is the only NP agent currently approved in the United States and the only ongoing example of NP pharmacogenetics, it is highly likely that the findings of the above studies will help quickly advance genetic targeting for any further NP agents that do come to market. This is true because of the shared effector and clearance mechanisms outlined above. As indicated earlier, there are several other NP agents under investigation as well an approved agent, carperitide, in Japan. If genetic targeting proves beneficial for recombinant BNP, it will be necessary to assess these other agents in terms of genetic interactions.
Conclusion
The NP system is a key counter-regulatory pathway in HF. There is substantial genetic variation in NP system components, and many of these variants have been shown to affect function or be associated with clinical phenotypes. How this variation can be used to genetically personalize medical therapy awaits further study. It is likely that functional variants will carry diagnostic/prognostic information themselves or modify the implications of NP testing. Ongoing studies will define the pharmacogenetics of nesiritide, which should be able to be quickly translated to other NP therapeutics.
References
- 1.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39(1):60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association. [Accessed 2004];Heart disease and stroke statistics—2005 Update. 2004 http://www.americanheart.org/presenter.jhtml?identifier=1928.
- 3.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 4.Popovic JR, Hall MJ. National hospital discharge survey. Hyattsville, Maryland: Center for Disease Control, National Center for Health Statistics; 1999. Apr 24, 2001. Report No.: 319. [Google Scholar]
- 5.Chen HH, Burnett JC., Jr The natriuretic peptides in heart failure: diagnostic and therapeutic potentials. Proc Assoc Am Physicians. 1999;111(5):406–416. doi: 10.1111/paa.1999.111.5.406. [DOI] [PubMed] [Google Scholar]
- 6.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 7.Scios JJ. Nesiritide Prescribing Information P0101208. [Google Scholar]
- 8.Suwa M, Seino Y, Nomachi Y, Matsuki S, Funahashi K. Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the ‘real world’ of therapy. Circ J. 2005;69(3):283–290. doi: 10.1253/circj.69.283. [DOI] [PubMed] [Google Scholar]
- 9.Nabel EG. Cardiovascular disease. N Engl J Med. 2003;349(1):60–72. doi: 10.1056/NEJMra035098. [DOI] [PubMed] [Google Scholar]
- 10.Evans WE, McLeod HL. Pharmacogenomics—drug disposition, drug targets, and side effects. N Engl J Med. 2003;348(6):538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 11.Lanfear DE, McLeod HL. Pharmacogenetics: using DNA to optimize drug therapy. Am Fam Physician. 2007;76(8):1179–1182. [PubMed] [Google Scholar]
- 12.Nakayama T. The genetic contribution of the natriuretic peptide system to cardiovascular diseases. Endocr J. 2005;52(1):11–21. doi: 10.1507/endocrj.52.11. [DOI] [PubMed] [Google Scholar]
- 13.LaPointe MC, Yang XP, Carretero OA, He Q. Left ventricular targeting of reporter gene expression in vivo by human BNP promoter in an adenoviral vector. Am J Physiol Heart Circ Physiol. 2002;283(4):H1439–H1445. doi: 10.1152/ajpheart.01090.2001. [DOI] [PubMed] [Google Scholar]
- 14.Stoupakis G, Klapholz M. Natriuretic peptides: biochemistry, physiology, and therapeutic role in heart failure. Heart Dis. 2003;5(3):215–223. doi: 10.1097/01.HDX.0000074517.30102.64. [DOI] [PubMed] [Google Scholar]
- 15.Koller KJ, Goeddel DV. Molecular biology of the natriuretic peptides and their receptors. Circulation. 1992;86(4):1081–1088. doi: 10.1161/01.cir.86.4.1081. [DOI] [PubMed] [Google Scholar]
- 16.Almirez R, Protter AA. Clearance of human brain natriuretic peptide in rabbits; effect of the kidney, the natriuretic peptide clearance receptor, and peptidase activity. J Pharmacol Exp Ther. 1999;289(2):976–980. [PubMed] [Google Scholar]
- 17.Rose RA, Giles WR. Natriuretic peptide C receptor (NPR-C) signaling in the heart and vasculature. J Physiol. 2008;586(2):353–366. doi: 10.1113/jphysiol.2007.144253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn M. Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res. 2003;93(8):700–709. doi: 10.1161/01.RES.0000094745.28948.4D. [DOI] [PubMed] [Google Scholar]
- 19.Sabrane K, Kruse MN, Fabritz L, et al. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Invest. 2005;115(6):1666–1674. doi: 10.1172/JCI23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtwick R, Gotthardt M, Skryabin B, et al. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA. 2002;99(10):7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellmers LJ, Knowles JW, Kim HS, Smithies O, Maeda N, Cameron VA. Ventricular expression of natriuretic peptides in Npr1(−/−) mice with cardiac hypertrophy and fibrosis. Am J Physiol Heart Circ Physiol. 2002;283(2):H707–H714. doi: 10.1152/ajpheart.00677.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellmers LJ, Scott NJ, Piuhola J, et al. Npr1-regulated gene pathways contributing to cardiac hypertrophy and fibrosis. J Mol Endocrinol. 2007;38(1–2):245–257. doi: 10.1677/jme.1.02138. [DOI] [PubMed] [Google Scholar]
- 23.Vellaichamy E, Kaur K, Pandey KN. Enhanced activation of pro-inflammatory cytokines in mice lacking natriuretic peptide receptor-A. Peptides. 2007;28(4):893–899. doi: 10.1016/j.peptides.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapoun AM, Liang F, O’Young G, et al. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myo-fibroblast conversion, proliferation, and inflammation. Circ Res. 2004;94(4):453–461. doi: 10.1161/01.RES.0000117070.86556.9F. [DOI] [PubMed] [Google Scholar]
- 25.John SW, Veress AT, Honrath U, et al. Blood pressure and fluid-electrolyte balance in mice with reduced or absent ANP. Am J Physiol. 1996;271(1 Pt 2):R109–R114. doi: 10.1152/ajpregu.1996.271.1.R109. [DOI] [PubMed] [Google Scholar]
- 26.Lopez MJ, Wong SK, Kishimoto I, et al. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378(6552):65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 27.Tamura N, Ogawa Y, Chusho H, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA. 2000;97(8):4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanfear DE, Stolker J, Marsh S, Rich MW, McLeod HL. Natriuretic Peptide Receptor 3 (NPR3) genotype modulates the relationship between B-Type Natriuretic Peptide (BNP) and left ventricular end-diastolic pressure. Therapy. 2006;3(6):765–771. [Google Scholar]
- 29.Lanfear DE, Marsh S, McLeod HL. Sequence variants of natriuretic peptide receptor C are common and their frequency differs between African Americans and European Americans. J Card Fail. 2004;10(4 Suppl):S59. [Google Scholar]
- 30.Rahmutula D, Nakayama T, Soma M, et al. Structure and polymorphisms of the human natriuretic peptide receptor C gene. Endocrine. 2002;17(2):85–90. doi: 10.1385/ENDO:17:2:085. [DOI] [PubMed] [Google Scholar]
- 31.Rahmutula D, Nakayama T, Soma M, et al. Systematic screening of type B human natriuretic peptide receptor gene polymorphisms and association with essential hypertension. J Hum Hypertens. 2001;15(7):471–474. doi: 10.1038/sj.jhh.1001199. [DOI] [PubMed] [Google Scholar]
- 32.Sezaki N, Ishimaru F, Tabayashi T, et al. The type 1 CD10/neutral endopeptidase 24.11 promoter: functional characterization of the 5′-untranslated region. Br J Haematol. 2003;123(1):177–183. doi: 10.1046/j.1365-2141.2003.04574.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang TJ, Larson MG, Levy D, et al. Heritability and genetic linkage of plasma natriuretic peptide levels. Circulation. 2003;108(1):13–16. doi: 10.1161/01.CIR.0000081657.83724.A7. [DOI] [PubMed] [Google Scholar]
- 34.Knowles JW, Erickson LM, Guy VK, Sigel CS, Wilder JC, Maeda N. Common variations in noncoding regions of the human natriuretic peptide receptor A gene have quantitative effects. Hum Genet. 2003;112(1):62–70. doi: 10.1007/s00439-002-0834-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama T, Soma M, Saito S, et al. Missense mutation of exon 3 in the type A human natriuretic peptide receptor gene is associated with myocardial infarction. Med Sci Monit. 2003;9(12):CR505–CR510. [PubMed] [Google Scholar]
- 36.Pitzalis MV, Sarzani R, Dessi-Fulgheri P, et al. Allelic variants of natriuretic peptide receptor genes are associated with family history of hypertension and cardiovascular phenotype. J Hypertens. 2003;21(8):1491–1496. doi: 10.1097/00004872-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama T, Soma M, Takahashi Y, Rehemudula D, Kanmatsuse K, Furuya K. Functional deletion mutation of the 5′-flanking region of type A human natriuretic peptide receptor gene and its association with essential hypertension and left ventricular hypertrophy in the Japanese. Circ Res. 2000;86(8):841–845. doi: 10.1161/01.res.86.8.841. [DOI] [PubMed] [Google Scholar]
- 38.Aoi N, Soma M, Nakayama T, et al. Variable number of tandem repeat of the 5′-flanking region of type-C human natriuretic peptide receptor gene influences blood pressure levels in obesity-associated hypertension. Hypertens Res. 2004;27(10):711–716. doi: 10.1291/hypres.27.711. [DOI] [PubMed] [Google Scholar]
- 39.Tremblay J, Hum DH, Sanchez R, et al. TA repeat variation, Npr1 expression, and blood pressure: impact of the Ace locus. Hypertension. 2003;41(1):16–24. doi: 10.1161/01.hyp.0000042664.75193.1b. [DOI] [PubMed] [Google Scholar]
- 40.Rubattu S, Bigatti G, Evangelista A, et al. Association of atrial natriuretic peptide and type a natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J Am Coll Cardiol. 2006;48(3):499–505. doi: 10.1016/j.jacc.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 41.Lanfear DE, Stolker JM, Marsh S, Rich MW, McLeod HL. Genetic variation in the B-type natiuretic peptide pathway affects BNP levels. Cardiovasc Drugs Ther. 2007;21(1):55–62. doi: 10.1007/s10557-007-6007-5. [DOI] [PubMed] [Google Scholar]
- 42.Palmer BR, Frampton CM, Richards AM, Cameron VA, Nakayama T. Absence of a NPR-A gene functional deletion allele in a postmyocardial infarction cohort from New Zealand. Circ Res. 2004;94(10):e86. [PubMed] [Google Scholar]
- 43.Nakayama T, Soma M, Mizutani Y, et al. A novel missense mutation of exon 3 in the type A human natriuretic peptide receptor gene: possible association with essential hypertension. Hypertens Res. 2002;25(3):395–401. doi: 10.1291/hypres.25.395. [DOI] [PubMed] [Google Scholar]
- 44.Rehemudula D, Nakayama T, Soma M, et al. Structure of the type B human natriuretic peptide receptor gene and association of a novel microsatellite polymorphism with essential hypertension. Circ Res. 1999;84(5):605–610. doi: 10.1161/01.res.84.5.605. [DOI] [PubMed] [Google Scholar]
- 45.Vassalle C, Andreassi MG, Prontera C, et al. Influence of ScaI and natriuretic peptide (NP) clearance receptor polymorphisms of the NP System on NP concentration in chronic heart failure. Clin Chem. 2007;53(11):1886–1890. doi: 10.1373/clinchem.2007.088302. [DOI] [PubMed] [Google Scholar]
- 46.Sarzani R, Dessi-Fulgheri P, Salvi F, et al. A novel promoter variant of the natriuretic peptide clearance receptor gene is associated with lower atrial natriuretic peptide and higher blood pressure in obese hypertensives. J Hypertens. 1999;17(9):1301–1305. doi: 10.1097/00004872-199917090-00010. [DOI] [PubMed] [Google Scholar]
- 47.Helisalmi S, Hiltunen M, Vepsalainen S, et al. Polymorphisms in neprilysin gene affect the risk of Alzheimer’s disease in Finnish patients. J Neurol Neurosurg Psychiatry. 2004;75(12):1746–1748. doi: 10.1136/jnnp.2004.036574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lilius L, Forsell C, Axelman K, Winblad B, Graff C, Tjernberg L. No association between polymorphisms in the neprilysin promoter region and Swedish Alzheimer’s disease patients. Neurosci Lett. 2003;337(2):111–113. doi: 10.1016/s0304-3940(02)01300-9. [DOI] [PubMed] [Google Scholar]
- 49.Nkeh B, Tiago A, Candy GP, et al. Association between an atrial natriuretic peptide gene polymorphism and normal blood pressure in subjects of African ancestry. Cardiovasc J S Afr. 2002;13(3):97–101. [PubMed] [Google Scholar]
- 50.Rubattu S, Stanzione R, Di Angelantonio E, et al. Atrial natriuretic peptide gene polymorphisms and risk of ischemic stroke in humans. Stroke. 2004;35(4):814–818. doi: 10.1161/01.STR.0000119381.52589.AB. [DOI] [PubMed] [Google Scholar]
- 51.Meirhaeghe A, Sandhu MS, McCarthy MI, et al. Association between the T–381C polymorphism of the brain natriuretic peptide gene and risk of type 2 diabetes in human populations. Hum Mol Genet. 2007;16(11):1343–1350. doi: 10.1093/hmg/ddm084. [DOI] [PubMed] [Google Scholar]
- 52.Kosuge K, Soma M, Nakayama T, et al. A novel variable number of tandem repeat of the natriuretic peptide precursor B gene’s 5′-flanking region is associated with essential hypertension among Japanese females. Int J Med Sci. 2007;4(3):146–152. doi: 10.7150/ijms.4.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiu C, Ingles J, Lind JM, Semsarian C. Mutation analysis of the natriuretic peptide precursor B (NPPB) gene in patients with hypertrophic cardiomyopathy. DNA Seq. 2006;17(5):392–395. doi: 10.1080/10425170600724998. [DOI] [PubMed] [Google Scholar]
- 54.Tiret L, Mallet C, Poirier O, et al. Lack of association between polymorphisms of eight candidate genes and idiopathic dilated cardiomyopathy: the CARDIGENE study. J Am Coll Cardiol. 2000;35(1):29–35. doi: 10.1016/s0735-1097(99)00522-7. [DOI] [PubMed] [Google Scholar]
- 55.Maisel AS, Clopton P, Krishnaswamy P, et al. Impact of age, race, and sex on the ability of B-type natriuretic peptide to aid in the emergency diagnosis of heart failure: results from the Breathing Not Properly (BNP) multinational study. Am Heart J. 2004;147(6):1078–1084. doi: 10.1016/j.ahj.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 56.Mak GS, DeMaria A, Clopton P, Maisel AS. Utility of B-natriuretic peptide in the evaluation of left ventricular diastolic function: comparison with tissue Doppler imaging recordings. Am Heart J. 2004;148(5):895–902. doi: 10.1016/j.ahj.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 57.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362(9380):316–322. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 58.Stolker JM, Rich MW. B-type natriuretic peptide provides independent and incremental value in predicting left ventricular end-diastolic pressure in elderly patients. Am J Geriatric Cardiol. 2005;14(2 Suppl):109. [Google Scholar]
- 59.Stolker JM, Rich MW. The combination of B-type natriuretic peptide and C-reactive protein provides incremental value in predicting outcomes among older patients referred for cardiac catheterization. Am J Geriatric Cardiol. 2005;14(2):109–110. doi: 10.1111/j.1076-7460.2007.05649.x. [DOI] [PubMed] [Google Scholar]
- 60.Drazner MH, de Lemos JA. Unexpected BNP levels in patients with advanced heart failure: a tale of caution and promise. Am Heart J. 2005;149(2):187–189. doi: 10.1016/j.ahj.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Troughton RW, Prior DL, Pereira JJ, et al. Plasma B-type natriuretic peptide levels in systolic heart failure: importance of left ventricular diastolic function and right ventricular systolic function. J Am Coll Cardiol. 2004;43(3):416–422. doi: 10.1016/j.jacc.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 62.Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135(5 Pt 1):825–832. doi: 10.1016/s0002-8703(98)70041-9. [DOI] [PubMed] [Google Scholar]
- 63.LaPointe MC. Molecular regulation of the brain natriuretic peptide gene. Peptides. 2005;26(6):944–956. doi: 10.1016/j.peptides.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 64.Waldo SW, Beede J, Isakson S, et al. Pro-B-type natriuretic peptide levels in acute decompensated heart failure. J Am Coll Cardiol. 2008;51(19):1874–1882. doi: 10.1016/j.jacc.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 65.Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343(4):246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 66.VMAC Study Group. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 67.Silver MA, Horton DP, Ghali JK, Elkayam U. Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. J Am Coll Cardiol. 2002;39(5):798–803. doi: 10.1016/s0735-1097(01)01818-6. [DOI] [PubMed] [Google Scholar]
- 68.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111(12):1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 69.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293(15):1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]