Abstract
The tissue cyst wall of Toxoplasma gondii is a stage-specific structure that is produced by modification of the bradyzoite-containing parasitophorous vacuole. It is a limiting membrane structure and is critically important for cyst survival and transmission of infection. Studies on the structure and function of the cyst wall should provide new therapeutic strategies for the elimination or prevention of latency during T. gondii infection. The membrane proteins of the T. gondii cyst are an important target for studies of the biochemical and immunological function(s) of the cyst. However, the components of the cyst membrane have been poorly characterized due to the difficulty of purification of these membrane proteins. We developed a lectin DBA (Dolichos biflorus) coated magnetic bead isolation method to isolate T. gondii cyst wall proteins. Our data suggests that this method can isolate cyst wall proteins from both in vitro cell culture or in vivo mouse brain derived tissue cysts. Antibodies to these isolated protein preparations were shown to localize to the cyst wall.
Keywords: Toxoplasma gondii, cyst wall, lectin, CST1, protein purification
1 Introduction
Toxoplasma gondii is an obligate intracellular parasite that can infect many mammals and birds throughout the world. It belongs to the phylum Apicomplexa that includes other important pathogens such as Plasmodium, Eimeria, Cyclospora, Babesia, and Cryptosporidium [33]. Infection with T. gondii is usually acquired orally either due to the ingestion of oocysts, produced by members of the cat family, or tissue cysts that are found in meat. Infection results in a transient acute phase, which can be associated with acute disease, caused by proliferative tachyzoites. This is followed by the formation of tissue cysts containing bradyzoites. The bradyzoite stage of T. gondii plays a critical role in maintenance of latent infection as well as the reactivation of chronic infections. In humans, progression of congenital T. gondii infection [23] as well as infection in immune suppressed individuals (e.g., patients with HIV infection) are usually due to the recrudescence of infection from tissue cysts [20]. It is hypothesized that in chronic toxoplasmosis bradyzoites regularly transform to tachyzoites, but that the tachyzoite growth is controlled by the immune system. In mice, new tissue cysts have been demonstrated to be formed during chronic infection [8,13]. Such a dynamic equilibrium between encysted and replicating forms would lead to recurrent antigenic stimulation, possibly accounting for the life-long persistence of antibody titers found in chronically infected hosts [10,11].
Bradyzoites appear structurally similar to tachyzoites by light microscopy, but ultrastructurally by electron microscopy bradyzoites have a more posterior nucleus, more honeycombed rhoptries than tachyzoites, and contain amylopectin granules [32,33]. A main feature of bradyzoites is that their parasitophorous vacuole becomes thickened forming the tissue cyst wall. The cyst wall is rich in carbohydrate and stains with various lectins [4, 16,39]. Previous studies have shown that tachyzoites and bradyzoites express stage specific antigens [32]. Several monoclonal antibodies have been developed that identify molecules specific to the tissue cyst [2,31,36,38]. The development of in vitro stage conversion models [1,27–29, 35] and reagents that recognize bradyzoites in vitro [2,14, 36] have facilitated studies of T. gondii cyst formation. For example, a gene knockout was preformed to identify the function of the bradyzoite specific molecule BAG1 and it was determined that this knockout resulted a phenotype change associated with decreased cyst numbers in an in vivo murine infection; however, tissue cysts were still formed both in vitro and in vivo and these cysts expressed other bradyzoite specific antigens [40].
The tissue cyst wall or bradyzoite parasitophorous vacuole membrane is elastic, thin (< 0.5 μm thick), faintly PAS positive, and argyrophic [24]. The tissue cyst wall is phase lucent by phase-contrast microscopy and the vacuole often contains an odd number of organisms (asynchronous division) that are club shaped [6,35]. Pale blue autofluorescence of the tissue cyst wall can be observed using UV (330 to 385 Å) [19]. The cyst wall appears to be composed of both host and parasite derived materials and lined by granular material which also fills up the space between the bradyzoites (particularly in mature cysts) [9,24,25]. Electron microscopy suggests that the cyst wall may be an active membrane interface. However, transport of molecules across this membrane has not been demonstrated. During development in astrocytes, the bradyzoite parasitophorous vacuole is surrounded by a layer of host cell intermediate filaments (glial fibrillary acidic protein) that limits the contact of the vacuole with host ER and mitochondria [15]; however, this material is not incorporated into the cyst wall.
In order to study the components of T. gondii cyst wall a primary requirement is the isolation of the cyst wall material from cyst tissue samples. A previous study from our laboratory demonstrated that one of the cyst wall specific molecules, CST1, is a glycoprotein that contains sugar residues that bind Dolichos biflorans lectin (DBA) [39]. Based on this finding, we developed a new approach employing DBA-magnetic beads for the purification of the cyst wall of T. gondii. Our study suggests that this method could successfully isolate cyst wall membrane material from in vitro or in vivo T. gondii cyst samples that could be used to raise antibodies to these proteins as well as for proteomic approaches for the characterization of these proteins using data available on the T. gondii genome (http://Toxodb.org). Studies on the T. gondii cyst wall should also help us to understand the process by which the cyst wall is formed and reveal the overall mechanism of bradyzoite differentiation.
2 Materials and methods
2.1 Parasite strain, tissue culture and, in vitro cyst formation
The R5 strain, an atovaquone-resistant PDS mutant of T. gondii, was utilized for this study [30]. Under stress condition such as pH change or sodium nitroprusside (SNP) treatment R5 strain tachyzoites readily differentiate into bradyzoites as demonstrated by the expression of bradyzoite specific antigens. Parasites were maintained by serial passage in confluent monolayers of human foreskin fibroblast cells (CCD-27SK; ATCC, Manassas, VA) or in BALB/3T3 murine fibroblasts (CCL 163; ATCC, Manassas, VA) grown in pH 7.1 Dulbecco’s Modified Eagle’s medium (Gibco-BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (Gibco-BRL, Gaithersburg, MD) and 1% penicillin-streptomycin (Gibco-BRL, Gaithersburg, MD). To induce bradyzoite differentiation and cyst formation in vitro, the culture medium was replaced with pH 8.1 medium 12 hours after inoculation and the pH 8.1 medium was changed daily [34]. For material utilized for antibody production in mice, the host cell line used was BALB/3T3 murine fibroblasts instead of human fibroblasts.
2.2 Preparation of DBA coated magnetic beads
4 × 108 Dynabead M-450 magnetic beads (Dynal Biotech, Brown Deer, WI) in a 1 mL suspension were washed twice with 2 mL of 0.1 M Phosphate buffer (Buffer A), resuspended in 0.5 mL 0.1 M Phosphate buffer and 0.5 mL of 1 mg/mL lectin DBA (Dolichos biflorus; E Y Laboratories, San Mateo, CA) solution was added to the bead suspension. The tube containing the DBA-magnetic bead mixture was placed on a rotary sample mixer and then incubated for 16–20 hours at 4°C. The final bead concentration was 4 × 107 beads/100 μL and the lectin/bead ratio was 12.5 μg DBA/107 beads. After incubation, the unlinked DBA was removed by washing the coated beads three times with buffer B (0.01 M Phosphate buffer, 0.15 M NaCl, 0.1% Bovine Serum Albumin, 0.02% Sodium Azide) using a magnetic stand to collect the beads during each wash (Dynal Biotech, Brown Deer, WI). After washing the DBA coated, magnetic beads were resuspended in 1 mL of Buffer B.
2.3 Isolation of the BPVM using DBA magnetic beads
Five to six days after the inoculation of T. gondii into eight 150 cm2 flasks containing fibroblasts under bradyzoite induction conditions, the medium was removed, cells were washed twice in phosphate buffered saline (PBS) (Gibco-BRL, Gaithersburg, MD), cells were then collected by gentle scraping in 10 mL of PBS per 150 cm2 flask, and then centrifuged at 1, 000 × g for 10 minutes at 4°C. The resultant cell pellets were then resuspended in 3 mL of cell disruption buffer containing 0.25 M Sucrose, 50 mM Tris-HCl, pH 7.5, 25 mM KCl, and 10 mM MgCl2 with a protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, IN) [22,26]. The infected host cell pellet was then disrupted in a ball-bearing homogenizer with a 0.0007 inch clearance, which does not disrupt T. gondii, but does disrupt the host cells [22,26]. The lysate was then centrifuged at 1, 000 × g for 10 minutes to remove parasites and debris. The membrane fraction and organelle complex in the resultant supernatant were collected by centrifugation at 13, 000 × g for 30 minutes using a Sorvall SS-34 rotor. The resulting membrane pellet was resuspended in 5.7 ml of pH 7.5 PBS containing 5% fetal bovine serum, 2 mM EDTA, and a protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, IN) and then 300 μL of the DBA coated magnetic beads were added to this suspension producing a final concentration of beads of 2 × 107/mL (equivalent to about 25 μg DBA/mL). This mixture was incubated at 4°C for 16 hours on a rotary sample mixer, the beads were gently washed four times with PBS supplemented with 5% FBS and then with PBS alone using a magnetic stand. Beads were then used directly for immunization or the bound cyst wall membrane was then eluted using sample buffer or water. We have called this cyst wall preparation the bradyzoite parasitophorous vacuole membrane (BPVM) as it is produced from in vitro culture.
2.4 Antibody production
In order to produce antibodies against T. gondii bradyzoite parasitophorous vacuole membrane (BPVM), BALB/c mice were immunized by intradermal injection with magnetic beads to which BPVM proteins isolated from infected BALB/3T3 murine fibroblasts were bound. Each mouse was injected with ~ 1.5 μg BPVM protein on beads using Hunter’s Titermax Gold (CytRx Corp., Norcross, Georgia) as an adjuvant, and the mice were boosted with the same antigen at four weeks after their initial immunization. Polyclonal antisera were obtained at three to four weeks after the second antigenic boost.
2.5 Electrophoresis and immunoblot analysis
BPVM was analyzed using SDS-PAGE electrophoresis performed using the methods described by Laemmli [17]. Samples were separated using 10% discontinuous gels (Micro Protean Gel Electrophoresis System; Bio-Rad, Hercules, California) and SDS-PAGE-resolved proteins were then transferred onto nitrocellulose membrane (Mini-Trans System; Bio-Rad, Hercules, California). The nitrocellulose blots were blocked overnight with 5% nonfat dried milk in PBS at 4°C, incubated at room temperature with a 1:500 dilution of anti-BPVM, or anti-CST1 (mAb 73.18 [36]) or anti-GRA5 (TG17-113, gift of Dr. M. Cesbron-Delauw, [18]) or anti-PDI (polyclonal rabbit antiserum, gift of Dr. R. Peek [21]) at room temperature for one hour, washed with PBS containing 0.1% Tween 20 (PBS-T) and then incubated with a goat anti-mouse IgG-IgM or goat anti-rabbit IgG-alkaline phosphate conjugate (Tropix, Bedford, Mass.) at 1 : 5, 000 or 1 : 10, 000 at room temperature. Antibody binding was then visualized using nitroblue tetrazolium-5-bromo-4-chloro-3-indolyphosphate (NBT-BCIP).
2.6 Electron microscopy
BPVM-DBA coated magnetic beads complexes were fixed in 2.5% (v/v) gluteraldehyde buffered with 0.1 m sodium cacodylate (pH 7.2) overnight at 4°C. Following fixation, the beads were rinsed in 0.1 M sodium cacodylate buffer, post-fixed in OsO4, dehydrated in a graded ethanol series, placed in propylene oxide, and embedded in Epon. Thin sections were placed on copper grids, stained with uranyl acetate/lead citrate, and then examined with a Phillips JEOL TEM operated at 80 kV.
2.7 Immunofluorescence assay (IFA)
T. gondii infected human fibroblast cultures in a 24-well plate were washed with PBS, fixed in 2% Formaldehyde in PBS for 30 minutes, and then washed 3 times for 1 minute in PBS. Following this, the cells were then permeabilized by incubating them with freshly made 0.2% Triton X-100 in PBS for 20 minutes, and then blocked with 1% BSA in PBS overnight at 4°C. After washing for 1 minute with PBS, they were incubated with anti-BPVM at a 1 : 50 dilution at 37°C for one hour in a moist chamber, washed three times for 1 minute each time with PBS, then incubated with fluorescein labeled anti-mouse IgG 1 : 500 for one our at 37°C in a moist chamber, and washed three times for 1 minute each time with PBS. The cells were then incubated with antiBAG1 rabbit polyclonal raised to recombinant BAG1 [37] at a 1 : 100 dilution at 37°C for one hour in a moist chamber, washed three times for 1 minute each time with PBS, then incubated with Texas red labeled anti-rabbit IgG 1 : 500 for one hour at 37°C in a moist chamber, and washed three times for 1 minute each time with PBS. The wells were overlaid with 2.5% DABCO (1,4-diazabicyclo-[2,2,2] octane) in PBS and viewed using a Nikon epifluorescence microscope.
3 RESULTS
3.1 Isolation of the BPVM using DBA-magnetic beads
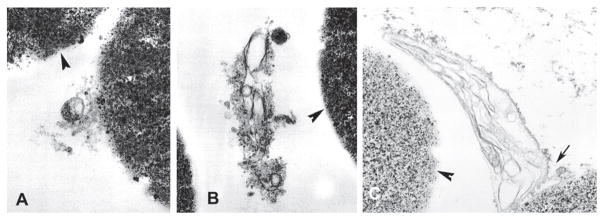
As described in the materials and methods section, a lectin DBA-magnetic bead isolation technique was developed to purify the cyst wall (i.e., BPVM) of T. gondii. The binding of fragments of the T. gondii BPVM to lectin DBA-magnetic beads was examined by electron microscopy. TEM of BPVM bound to DBA-magnetic beads demonstrated material consistent with membrane fragments from T. gondii infected BALB/3T3 cells (Figures 1(A) and 1(B)). These bound fragments were not seen when uninfected BALB/3T3 were used as starting material for BPVM purification. Similar images were seen when in vivo T. gondii tissue cysts purified from infected mouse brains [39] were used as starting material for this DBA-magnetic beads isolation method (Figure 1(C)).
Figure 1.
Transmission electron microscopy of the purification of bradyzoite parasitophorous vacuole membrane (BPMV) on DBA coated magnetic beads. Panels A and B demonstrate membranes from in vitro cultures bound to lectin coated beads (arrowheads). Panel C demonstrates area of binding of tissue cyst wall (i.e., bradyzoite parasitophorous membrane) (arrow) from tissue cysts purified from mouse brain to the lectin coated bead (arrowhead) surface.
As demonstrated in Figure 2(A), immunoblot of the BPVM using various antibodies demonstrated that the BPVM contained both GRA5, recognized by mAb TG17.113 (lane 1) and CST1, recognized by mAb 73.18 (lane 3). Both of these proteins have been previously reported to be present on the cyst wall by EM and IFA techniques [7,39]. This material did not react with anti-BAG1 (data not shown). This result suggested that the DBA-magnetic bead method did indeed enrich material that contained cyst wall fragments. We had previously determined by mass spectrometry (mass mapping) that protein disulfide isomerase (PDI) was present in a gel slice containing a 50 kDA band seen on a coomassie blue stained SDS-PAGE of the proteins of the BPVM isolated by these DBA beads (Zhang Y, Halonen SK, Weiss LM. Characterization of components of the cyst wall in Toxoplasma gondii, presented at the 7th International Congress on Toxoplasmosis, Tarrytown, NY, 2003). Anti-TgPDI reacted with BPVM isolated by the DBA magnetic beads (Figure 2, lane 2).
Figure 2.
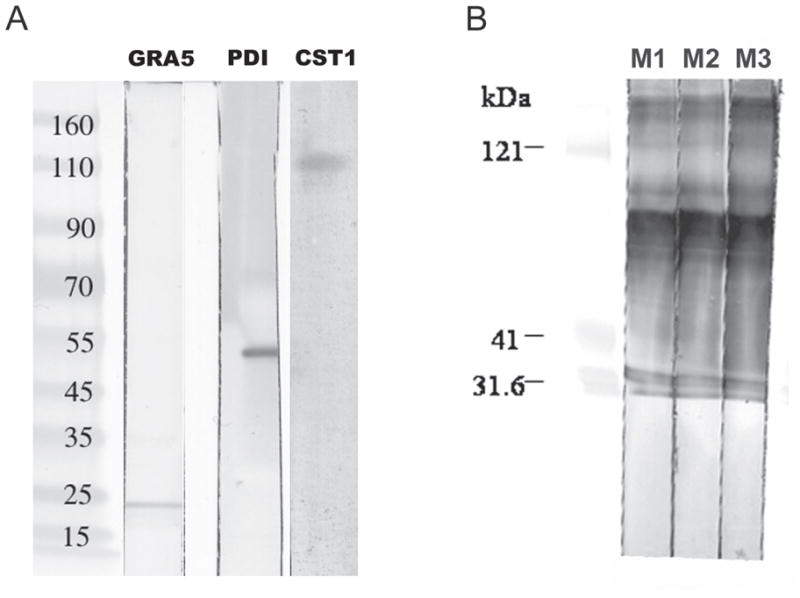
Immunoblot analysis of DBA magnetic bead purified bradyzoite parasitophorous vacuole membrane (BPVM). Panel A: antibody to GRA5 (TG17-113) reacts with a band of about 21 kDa, antibody to Protein Disulfide Isomerase (PD) to an band of about 55 kDa, and antibody to CST1 (73.18) reacts with a band of about 116 kDa (identical to the band seen with DBA lectin [39]). Panel B: mice immunized with purified bradyzoite parasitophorous membranes react on immunoblot to multiple bands from this material (lanes M1, M2, and M3 represent sera from three different mice that were immunized with purified BPVM).
3.2 Polyclonal antisera against BPVM isolated by DBA-magnetic beads
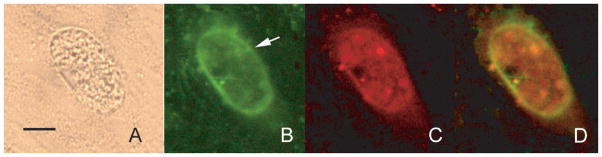
The DBA-magnetic bead isolated BPVM from T. gondii infected BALB/3T3 cells was used to immunize three BALB/c mice. The antisera obtained from these mice when examined by immunoblot (Figure 2(B)) demonstrated reactivity with several antigens in the BPVM preparation (at least 7 major bands can be seen in this immunoblot). Figure 3 demonstrates the immunofluorescence microscopy results using this anti-BPVM polyclonal mouse serum to examine human fibroblasts infected with Toxoplasma gondii R5 parasites at pH 8.0. Anti-BPVM reacted with the cyst wall of the vacuole (Figure 3 arrow). Staining of these organisms with BAG1 demonstrated that these vacuoles contained bradyzoites. Anti-BPVM only reacted with vacuoles containing bradyzoites (i.e., BAG1 reactive) and not with vacuoles containing tachyzoites (i.e., there was no reaction with vacuoles that were negative for BAG1). Some background fluorescence can be seen and probably is related to host proteins that can be bound by DBA and would copurify with BPVM on the DBA magnetic beads.
Figure 3.
Reactivity of anti-BPVM with T. gondii bradyzoite vacuoles in vitro. Panel A: phase contrast microscopy (40× objective) of T. gondii in a human fibroblast cell demonstrating a refractile vacuole membrane. The bar is 15 μm. Panel B: reactivity of this vacuole (arrow) with polyclonal anti-BPVM mouse antiserum detected with fluorescein antimouse IgG demonstrating staining of the vacuole membrane. Panel C: reactivity of the vacuole with anti-BAG1 rabbit antibody detected with Texas red antirabbit IgG demonstrating that this vacuole contains bradyzoites. Panel D is an overlay of Panels B and C demonstrating the reactivity of anti-BPVM with the vacuole membrane.
4 Discussion
The T. gondii cyst wall membrane is thought to be important in maintaining the integrity of the cyst in host cells for long periods. The cyst wall consists of a highly invaginated outer membrane underlaid with a dense osmiophilic matrix containing membranous vesicles. It measures 200–850 nm in thickness. The tissue cyst wall is a modification of the limiting membrane of the parasitophorous vacuole and is elastic, argyrophilic, and PAS reactive [5,25,32]. This modification may limit communication of this intracellular parasite with its host cells. Additionally, the cyst wall probably limits antigen presentation to the host contributing to the persistence of this intracellular parasite. That modification of the parasitophorous vacuole into a developing cyst wall is an early event that accompanies bradyzoite differentiation [3,12,32]. The cyst wall and matrix probably protect bradyzoites from harsh environmental conditions and also provide a physical barrier to host immune defenses. Studies on the structure and function of the cyst wall may suggest novel therapeutic strategies for the elimination or prevention of latency during T. gondii infection.
The tissue cyst wall of T. gondii binds both Dolichos biflorus (DBA) and succinylated wheat-germ agglutinin (S-WGA) (Boothroyd et al., 1997) [4,16,39]. This binding can be inhibited by competition with the sugar haptens N-acetylgalactasamine (GalNAc) for DBA and N-acetylglucosamine (GlcNAc) for S-WGA [4]. Treatment with chitinase disrupts the cyst wall and eliminates S-WGA binding, consistent with the presence of chitin in this structure [4]. Binding of DBA is also seen on the cyst wall of cysts of the related coccidian parasite Neospora caninum developing in vitro [37]. Our previous study demonstrated that DBA binds a specific T. gondii cyst wall molecule CST1 [39]. As demonstrated in the present study, this DBA binding can be used as the basis for a technique to isolate Toxoplasma gondii cyst wall (bradyzoite parasitophorous membrane) proteins. The cyst wall proteins isolated by this method are antigenic and could be used to produce antisera that reacted by IFA with in vitro derived bradyzoite parasitophorous vacuoles. While this method will also purify host cell proteins (or membrane fragments) that bind DBA as well as any other T. gondii proteins that bind DBA, it represents an approach that allows enrichment of T. gondii proteins associated with the cyst wall (i.e., the BPVM).
There has been a surge of interest in parasite proteomics with an increasing number of articles and journals dedicated to the subject. This has been spurred on the completion of various parasite genomes. The T. gondii genome has been completed and is available at www.toxodb.org. Gene prediction on this genome data has recently been refined and these predictions allow the use of proteomic techniques for the characterization of complex protein mixtures. This isolation technique should be useful for the development of a proteome of the cyst wall facilitating proteomic approaches employing mass spectrometry in the characterization of this important T. gondii structure.
Acknowledgments
This work was supported by NIH Grant AI 39454 (LMW). The authors are thankful to the Analytical Imaging Facility of the Albert Einstein College of Medicine for assistance on transmission electron microscopy and fluorescence microscopy.
References
- 1.Bohne W, Heesemann J, Gross U. Coexistence of heterogeneous populations of Toxoplasma gondii parasites within parasitophorous vacuoles of murine macrophages as revealed by a bradyzoite-specific monoclonal antibody. Parasitol Res. 1993;79:485–487. doi: 10.1007/BF00931588. [DOI] [PubMed] [Google Scholar]
- 2.Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohne W, Holpert M, Gross U. Stage differentiation of the protozoan parasite Toxoplasma gondii. Immunobiology. 1999;201:248–254. doi: 10.1016/S0171-2985(99)80065-5. [DOI] [PubMed] [Google Scholar]
- 4.Boothroyd JC, Black M, Bonnefoy S, Hehl A, Knoll LJ, Manger ID, et al. Genetic and biochemical analysis of development in Toxoplasma gondii. Philos Trans R Soc Lond B Biol Sci. 1997;352:1347–1354. doi: 10.1098/rstb.1997.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dzierszinski F, Nishi M, Ouko L, Roos DS. Dynamics of Toxoplasma gondii differentiation. Eukaryot Cell. 2004;3:992–1003. doi: 10.1128/EC.3.4.992-1003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson DJ. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int J Parasitol. 2004;34:347–360. doi: 10.1016/j.ijpara.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson DJ, Huskinson-Mark J, Araujo FG, Remington JS. A morphological study of chronic cerebral toxoplasmosis in mice: comparison of four different strains of Toxoplasma gondii. Parasitol Res. 1994;80:493–501. doi: 10.1007/BF00932696. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson DJP, Hutchison WM. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol Res. 1987;73:483–491. doi: 10.1007/BF00535321. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson DJP, Hutchison WM, Pettersen E. Tissue cyst rupture in mice chronically infected with Toxoplasma gondii. An immunocytochemical and ultrastructural study. Parasitol Res. 1989;75:599–603. doi: 10.1007/BF00930955. [DOI] [PubMed] [Google Scholar]
- 11.Frenkel JK, Escajadillo A. Cyst rupture as a pathogenic mechanism of toxoplasmic encephalitis. Am J Trop Med Hyg. 1987;36:517–522. doi: 10.4269/ajtmh.1987.36.517. [DOI] [PubMed] [Google Scholar]
- 12.Gross U, Bohne W, Soête M, Dubremetz JF. Developmental differentiation between Tachyzoites and Bradyzoites of Toxoplasma gondii. Parasitol Today. 1996;12:30–33. doi: 10.1016/0169-4758(96)80642-9. [DOI] [PubMed] [Google Scholar]
- 13.Gross U, Bohne W, Windeck T, Heesemann J. New views on the pathogenesis and diagnosis of toxoplasmosis. Immun Infekt. 1992;20:151–155. [PubMed] [Google Scholar]
- 14.Gross U, Bormuth H, Gaissmaier C, Dittrich C, Krenn V, Bohne W, et al. Monoclonal rat antibodies directed against Toxoplasma gondii suitable for studying tachyzoite-bradyzoite interconversion in vivo. Clin Diagn Lab Immunol. 1995;2:542–548. doi: 10.1128/cdli.2.5.542-548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halonen SK, Weiss LM, Chiu FC. Association of host cell intermediate filaments with Toxoplasma gondii cysts in murine astrocytes in vitro. Int J Parasitol. 1998;28:815–823. doi: 10.1016/s0020-7519(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 16.Knoll LJ, Boothroyd JC. Molecular biology’s lessons about Toxoplasma development: stage-specific homologs. Parasitology Today. 1998;14:490–493. doi: 10.1016/s0169-4758(98)01347-7. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Lecordier L, Mercier C, Torpier G, Tourvieille B, Darcy F, Liu JL, et al. Molecular structure of a Toxoplasma gondii dense granule antigen (GRA 5) associated with the parasitophorous vacuole membrane. Mol Biochem Parasitol. 1993;59:143–153. doi: 10.1016/0166-6851(93)90015-p. [DOI] [PubMed] [Google Scholar]
- 19.Lei Y, Davey M, Ellis J. Autofluorescence of Toxoplasma gondii and Neospora caninum cysts in vitro. J Parasitol. 2005;91:17–23. doi: 10.1645/GE-355R. [DOI] [PubMed] [Google Scholar]
- 20.Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 21.Meek B, Back JW, Klaren VN, Speijer D, Peek R. Protein disulfide isomerase of Toxoplasma gondii is targeted by mucosal IgA antibodies in humans. FEBS Lett. 2002;522:104–108. doi: 10.1016/s0014-5793(02)02911-3. [DOI] [PubMed] [Google Scholar]
- 22.Ossorio PN, Dubremetz JF, Joiner KA. A soluble secretory protein of the intracellular parasite Toxoplasma gondii associates with the parasitophorous vacuole membrane through hydrophobic interactions. J Biol Chem. 1994;269:15350–15357. [PubMed] [Google Scholar]
- 23.Remington JS, McLeod R, Desmonts G. Toxoplasmosis. In: Remington JS, Klein JO, editors. Infectious Diseases of the Fetus and Newborn Infant. W. B. Saunders Company; Philadephia: 1995. pp. 140–267. [Google Scholar]
- 24.Sims TA, Hay J, Talbot IC. Host-parasite relationship in the brains of mice with congenital toxoplasmosis. J Pathol. 1988;156:255–261. doi: 10.1002/path.1711560311. [DOI] [PubMed] [Google Scholar]
- 25.Sims TA, Hay J, Talbot IC. An electron microscope and immunohistochemical study of the intracellular location of Toxoplasma tissue cysts within the brains of mice with congenital toxoplasmosis. Br J Exp Pathol. 1989;70:317–325. [PMC free article] [PubMed] [Google Scholar]
- 26.Sinai AP, Webster P, Joiner KA. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J Cell Sci. 1997;110:2117–2128. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- 27.Soête M, Camus D, Dubremetz JF. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–370. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 28.Soête M, Dubremetz JF. Toxoplasma gondii: kinetics of stage-specific protein expression during tachyzoite-bradyzoite conversion in vitro. Curr Top Microbiol Immunol. 1996;219:76–80. [PubMed] [Google Scholar]
- 29.Soete M, Fortier B, Camus D, Dubremetz JF. Toxoplasma gondii: kinetics of bradyzoite-tachyzoite interconversion in vitro. Exp Parasitol. 1993;76:259–264. doi: 10.1006/expr.1993.1031. [DOI] [PubMed] [Google Scholar]
- 30.Tomavo S, Boothroyd JC. Interconnection between organellar functions, development and drug resistance in the protozoan parasite, Toxoplasma gondii. Int J Parasitol. 1995;25:1293–1299. doi: 10.1016/0020-7519(95)00066-b. [DOI] [PubMed] [Google Scholar]
- 31.Tomavo S, Fortier B, Soete M, Ansel C, Camus D, Dubremetz JF. Characterization of bradyzoite-specific antigens of Toxoplasma gondii. Infect Immun. 1991;59:3750–3753. doi: 10.1128/iai.59.10.3750-3753.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss LM, Kim K. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci. 2000;5:391–405. doi: 10.2741/weiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss LM, Kim K, editors. The Model Apicomplexan: Perspectives and Methods. Academic Press; London: 2007. Toxoplasma gondii. [Google Scholar]
- 34.Weiss LM, Laplace D, Takvorian P, Tanowitz HB, Wittner M. The association of the stress response and Toxoplasma gondii bradyzoite development. J Eukaryot Microbiol. 1996;43:120S. doi: 10.1111/j.1550-7408.1996.tb05036.x. [DOI] [PubMed] [Google Scholar]
- 35.Weiss LM, Laplace D, Takvorian PM, Tanowitz HB, Cali A, Wittner M. A cell culture system for study of the development of Toxoplasma gondii bradyzoites. J Eukaryot Microbiol. 1995;42:150–157. doi: 10.1111/j.1550-7408.1995.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 36.Weiss LM, LaPlace D, Tanowitz HB, Wittner M. Identification of Toxoplasma gondii bradyzoite-specific monoclonal antibodies. J Infect Dis. 1992;166:213–215. doi: 10.1093/infdis/166.1.213. [DOI] [PubMed] [Google Scholar]
- 37.Weiss LM, Ma YF, Halonen S, McAllister MM, Zhang YW. The in vitro development of Neospora caninum bradyzoites. Int J Parasitol. 1999;29:1713–1723. doi: 10.1016/s0020-7519(99)00130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang YW, Fraser A, Balfour AH, Wreghitt TG, Gray JJ, Smith JE. Serological reactivity against cyst and tachyzoite antigens of Toxoplasma gondii determined by FAST-ELISA. J Clin Pathol. 1995;48:908–911. doi: 10.1136/jcp.48.10.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang YW, Halonen SK, Ma YF, Wittner M, Weiss LM. Initial characterization of CST1, a Toxoplasma gondii cyst wall glycoprotein. Infect Immun. 2001;69:501–507. doi: 10.1128/IAI.69.1.501-507.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang YW, Kim K, Ma YF, Wittner M, Tanowitz HB, Weiss LM. Disruption of the Toxoplasma gondii bradyzoite-specific gene BAG1 decreases in vivo cyst formation. Mol Microbiol. 1999;31:691–701. doi: 10.1046/j.1365-2958.1999.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]