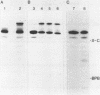
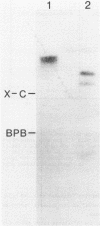
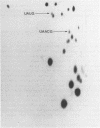
Abstract
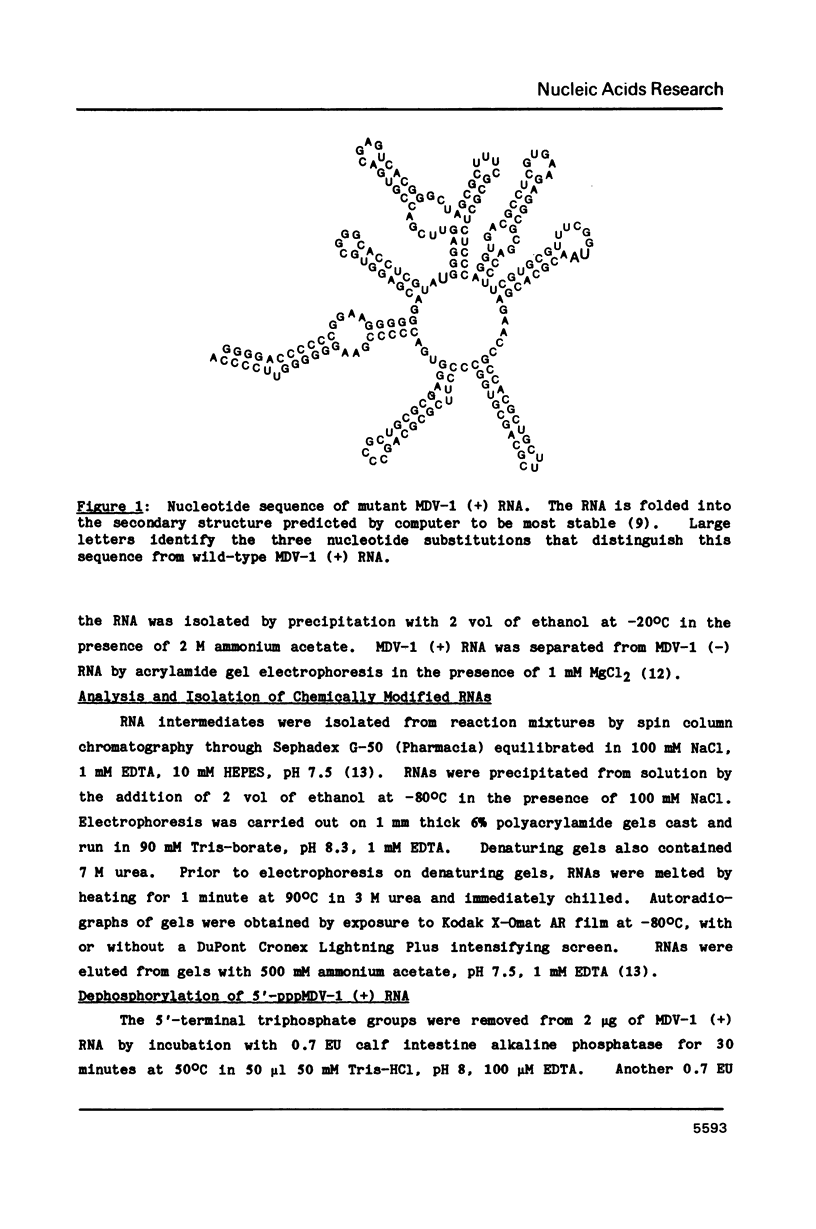
The replacement of reporter groups, such as fluorescent molecules or enzymes, by an amplifiable reporter should lead to bioassays of greatly increased sensitivity, since a very large number of copies of the reporter can be accumulated in a short time. Midivariant RNA is an appropriate reporter, since it is autocatalytically replicated by Q beta RNA polymerase in vitro. This RNA can be amplified exponentially, with a population doubling time of 36 seconds, resulting in the synthesis of 10(6) copies of each molecule in 12 minutes. We have used chemical methods to attach biotin to the 5' terminus of midivariant RNA via a disulfide linker. This biotinylated RNA combines with avidin to give a product that is readily purified by gel electrophoresis. The RNA-biotin-avidin adduct, and the RNA released from it by reductive cleavage of the linker arm, replicate normally. The RNA-biotin-avidin adduct should be a suitable reporter for a variety of replication-assisted bioassays involving biotinylated antibodies or biotinylated nucleic acid probes.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K., Kuo C. H., August J. T. Replication of RNA viruses. 8. Direction of chain growth in the Q-beta RNA polymerase reaction. J Mol Biol. 1969 Mar 28;40(3):445–455. doi: 10.1016/0022-2836(69)90164-8. [DOI] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Detection of specific DNA sequences with short biotin-labeled probes. DNA. 1985 Aug;4(4):327–331. doi: 10.1089/dna.1985.4.327. [DOI] [PubMed] [Google Scholar]
- Chu B. C., Wahl G. M., Orgel L. E. Derivatization of unprotected polynucleotides. Nucleic Acids Res. 1983 Sep 24;11(18):6513–6529. doi: 10.1093/nar/11.18.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruna I., Spiegelman S. Autocatalytic synthesis of a viral RNA in vitro. Science. 1965 Nov 12;150(3698):884–886. doi: 10.1126/science.150.3698.884. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Mills D. R., Kramer F. R., Spiegelman S. A replicating RNA molecule suitable for a detailed analysis of extracellular evolution and replication. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3038–3042. doi: 10.1073/pnas.69.10.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer F. R., Mills D. R., Cole P. E., Nishihara T., Spiegelman S. Evolution in vitro: sequence and phenotype of a mutant RNA resistant to ethidium bromide. J Mol Biol. 1974 Nov 15;89(4):719–736. doi: 10.1016/0022-2836(74)90047-3. [DOI] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levisohn R., Spiegelman S. The cloning of a self-replicating RNA molecule. Proc Natl Acad Sci U S A. 1968 Jul;60(3):866–872. doi: 10.1073/pnas.60.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. R., Dobkin C., Kramer F. R. Template-determined, variable rate of RNA chain elongation. Cell. 1978 Oct;15(2):541–550. doi: 10.1016/0092-8674(78)90022-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]