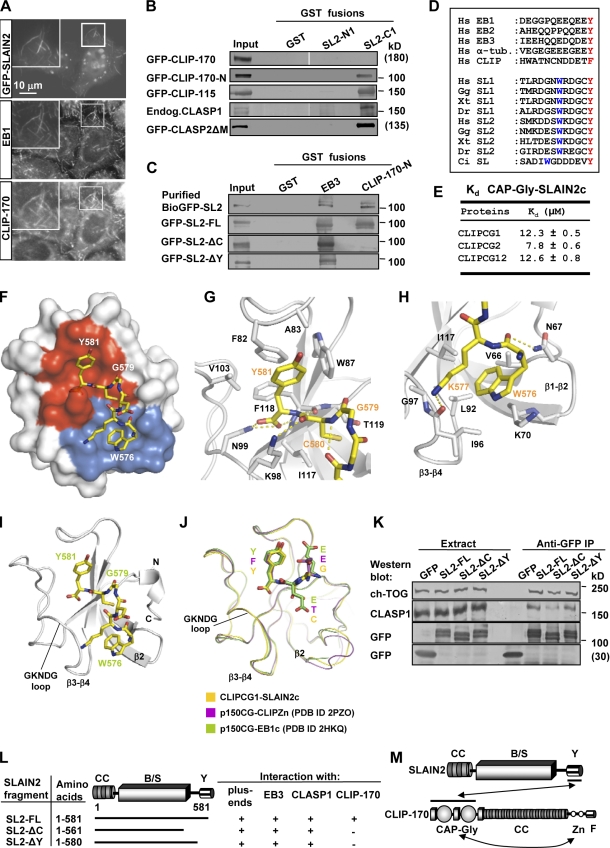
Figure 2.
SLAIN2 interacts with CLIPs and CLASPs. (A) HeLa cells were transiently transfected with GFP-SLAIN2, fixed, and labeled with the indicated antibodies. Insets show enlargements of the boxed areas. (B and C) GST pull-down assays were performed with the indicated GST fusions and lysates of untransfected HeLa cells or cells expressing the indicated GFP fusions (SLAIN2 is abbreviated as SL2). Western blots were performed using the antibodies against GFP or CLASP1. The top lane of C shows a GST pull-down assay with BioGFP-SLAIN2 purified from HEK293 cells. White lines indicate that intervening lanes have been spliced out. (D) Alignment of the C-terminal tails of human EB1, EB2, EB3, α-tubulin (α-tub.), CLIP-170, and SLAIN1 and SLAIN2 from different species. Hs, Homo sapiens; Gg, Gallus gallus; Xt, Xenopus tropicalis; Dr, Danio rerio; Ci, Ciona intestinalis. The conserved C-terminal aromatic and the tryptophan residues are highlighted. (E) Equilibrium dissociation constants obtained by ITC for the complexes of the human SLAIN2 peptide (SLAIN2c) with either the first (CLIPCG1), second (CLIPCG2), or both (CLIPCG12) CAP-Gly domains of CLIP-170. (F) Overall view of the heterodimeric complex formed between CLIPCG1 (surface representation) and SLAIN2c (sticks representation). Contact modes A and B are shown in red and blue, respectively. (G and H) Close up views of the interaction network seen in the complex formed between SLAIN2c (yellow carbon atoms) and CLIPCG1 (gray carbon atoms) in the cartoon (main chain) and sticks (contacting residues) representation. Panels G and H depict contact modes A and B, respectively. (I and J) Overall view of the heterodimeric complex formed between CLIPCG1 (ribbon representation) and SLAIN2c (sticks representation; I) and superposition of complexes formed between CAP-Gly domains and C-terminal tyrosine or phenylalanine-containing sequence regions (J). For simplicity, only the last three C-terminal residues of the respective CAP-Gly ligands are shown in sticks representation. (K) IP with anti-GFP antibodies from extracts of HeLa cells expressing GFP or GFP-SLAIN2 fusions were analyzed by Western blotting with the indicated antibodies. (L) Mapping of the SLAIN2 interaction site with CLIP-170, EB3, CLASP1, and MT plus ends. (M) Schematic overview of the SLAIN2–CLIP-170 interaction. CC, coiled-coil; B/S, basic and serine rich; Y, C-terminal tyrosine; Zn, zinc knuckles; F, C-terminal phenylalanine; Endog., endogenous; PDB, Protein Data Bank.