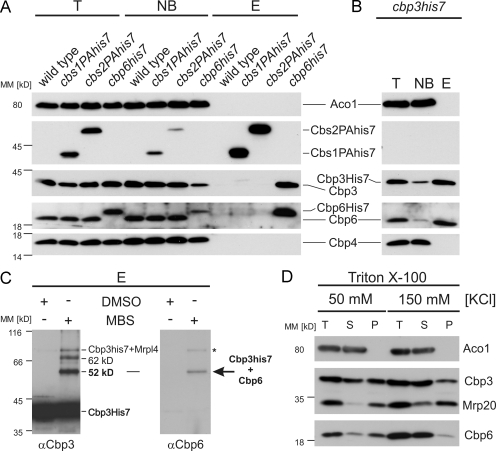
Figure 4.
Cbp3 forms a complex with Cbp6. (A) Native purification of Cbs1, Cbs2, and Cbp6 complexes. Cbs1 and Cbs2 were equipped with C-terminal ProteinAHis7 tags to allow sensitive detection as well as purification of the proteins via metal affinity chromatography, whereas Cbp6 was expressed with a C-terminal His7 tag. Triton X-100 lysates of the indicated mitochondria were subjected to Ni-NTA chromatography, and the resulting fractions were analyzed by Western blotting. E, elution after Ni-NTA purification; NB, 20% of unbound material after Ni-NTA purification; PAHis7 tag, ProteinA-heptahistidine tag; T, 20% total before Ni-NTA purification. (B) Cbp6 is efficiently purified with Cbp3His7. Native purification of the Cbp3 complex from cbp3his7 mitochondria was performed as described in A. (C) Cbp3 and Cbp6 can be cross-linked to each other. Mitochondria harboring Cbp3His7 were incubated with the cross-linker MBS or a control (DMSO). Cbp3 and cross-linked proteins were purified as described in Fig. 1 B. The elution fractions were analyzed by Western blotting using antibodies against Cbp3 (left) and Cbp6 (right). The asterisk indicates the cross-link of Mrpl4 to Cbp3His7. (D) Cbp6 co-migrates with mitochondrial ribosomes in a salt-sensitive manner. Triton X-100 lysates of wild-type mitochondria were fractionated as described in Fig. 1 C. MM, molecular mass; P, pellet; S, supernatant; T, 100% total before ribosome fractionation.