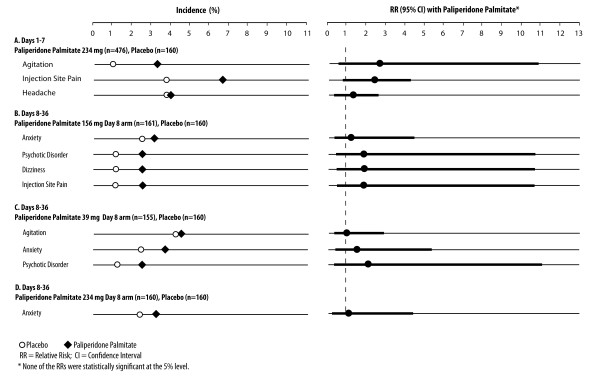
Figure 4.
Adverse Events in ≥2% of Paliperidone Palmitate and in a Higher Percentage of Paliperidone Palmitate than Placebo Subjects. Adverse events meeting these criteria during Days 1 to 7 are shown in Panel A for subjects received paliperidone palmitate 234 mg Day 1 (rates and relative risks versus placebo with 95% CIs); none were statistically significant as determined by 95% CIs. Adverse events that met these criteria during Days 8 to 36 are shown in Panel B for the paliperidone palmitate 156 mg Day 8 group, Panel C for the 39 mg Day 8 group, and Panel D for the 234 mg Day 8 group. None were statistically significant as determined by 95% CIs.