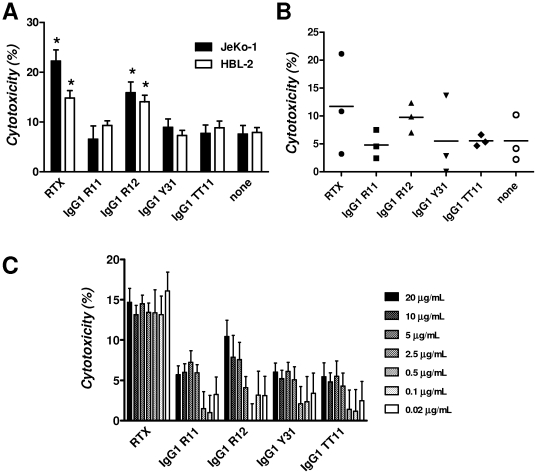
Figure 9. ADCC.
A bioluminescent intracellular protease detection assay was used to quantify the ADCC potency of IgG1 R11, R12, and Y31 in comparison to IgG1 TT11 (negative control) and rituximab (RTX; positive control; all at 5 µg/mL) toward (A) MCL lines JeKo-1 and HBL-2 and (B) PBMC from patients CLL-2, CLL-3, and CLL-4. NK cells isolated from PBMC of a healthy volunteer were used as effector cells at an effector-to-target cell ratio of 25∶1. In (A), specific cytotoxicity is shown as columns with error bars to indicate mean and standard deviation values of side-by-side triplicates. Stars indicate significant differences (p<0.05) to both the negative control (IgG1 TT11) and the background (none). In (B), specific cytotoxicity toward PBMC from the three individual patients is shown with mean values indicated by horizontal bars. (C) Using the same assay and mAbs, ADCC potency toward HBL-2 cells was compared over a concentration range of 0.02 to 20 µg/mL. NK cells isolated from PBMC of a different healthy volunteer were used at an effector-to-target cell ratio of 20∶1. Columns indicate mean values and error bars indicate standard deviation values of side-by-side triplicates.