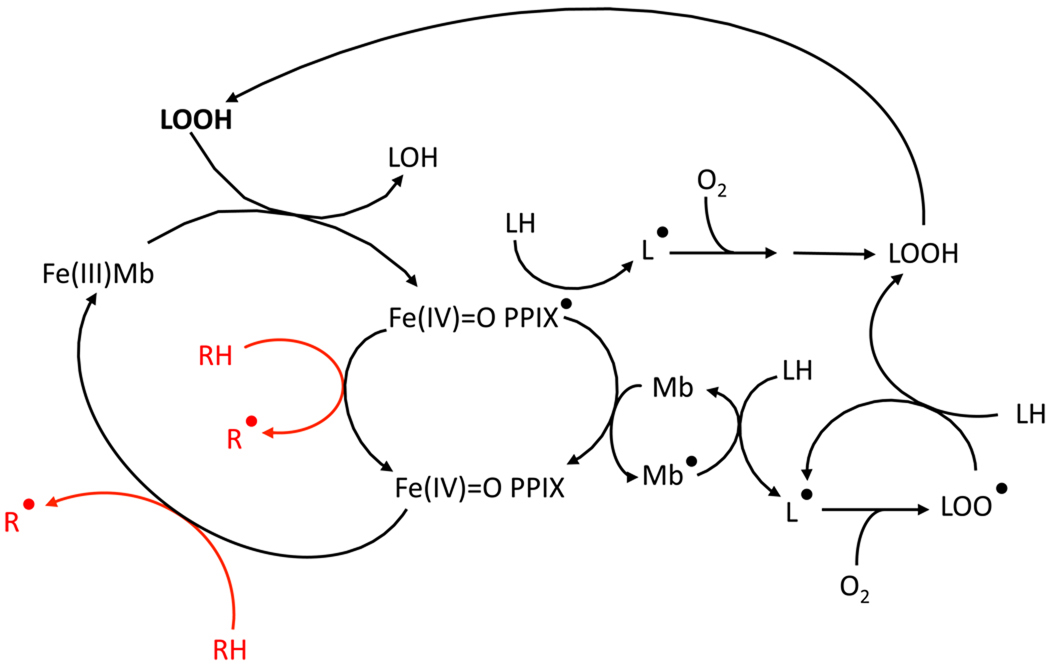
Figure 1. Mechanism for Mb-induced lipid peroxidation and its inhibition by acetaminophen.
Oxidation of ferric Mb (Fe(III)Mb) by a lipid hydroperoxide (LOOH) yields the ferryl Mb protoporphyrin radical (Fe(IV)=O PPIX •). This radical can delocalize to the protein to form a globin radical (Mb •), which can in turn catalyze lipid peroxidation by abstracting an electron from a lipid (LH). The lipid radical (L •) can then react to molecular oxygen to form the peroxyl radical (LOO •), which can propagate lipid peroxidation. Reducing co-substrates such as ApAP (RH) reduce the ferryl Mb back to its ferric state, thus inhibiting Mb-catalyzed lipid peroxidation.