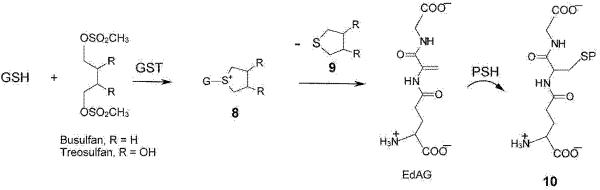
Figure 4. Formation of glutathione S-conjugates of busulfan and facile decomposition to a dehydroalanyl analogue of GSH.
Busulfan (R = H) reacts with GSH in a reaction that occurs spontaneously under physiological conditions, but which is accelerated by GSTs, to form the glutathione S-conjugate 8. This glutathione S-conjugate contains an unusual sulfonium moiety (most glutathione S-conjugates are thioethers). The cyclic sulfonium contains an exceptionally good leaving group. Thus, tetrahydrothiophene (9) is readily eliminated generating a dehydroalanyl analogue of GSH (γ-glutamyldehydroalanylglycine, EdAG). Michael addition of GSH to this compound results in the formation of GSG, a GSSG analogue in which the reducible disulfide bond is replaced by a non-reducible thioether. We suggest that the reaction of EdAG with an exposed cysteine residue of a susceptible residue (PSH) may result in irreversible glutathionylation, generating a protein linked to a glutathione molecule through a non-reducible thioether bond (10). This reaction is identical to that depicted in figure 2 (6 → 7), except that the Michael acceptor and donor are reversed. Although the possible formation of a sulfonium conjugate of treosulfan has not yet been investigated, given the similarity in structure of treosulfan to busulfan, we predict that it will form under in vivo conditions.