Abstract
Objective. STR/ORT mice provide a well-known model for murine idiopathic OA, with histological joint lesions resembling those of human OA. This model was used to investigate protective effects of the dipeptide aspartyl-phenylalanine-1-methyl ester (Asp-Phe-OMe or aspartame) via the oral route vs a regular diet.
Methods. STR/ORT mice were housed individually and fed diets with or without Asp-Phe-OMe (4 mg/kg), after weaning at the age of 3 weeks, until 15 months of age (average of 20 animals per group). The study groups were kept blinded to the investigators, who measured food consumption and body weight and performed gait mobility tests. Radiographic scans were also performed at regular time intervals to evaluate differential radiographic anomalies associated with progress of OA in response to oral Asp-Phe-OMe therapy.
Results. The Asp-Phe-OMe-fed animals presented a pattern of significantly delayed disease onset. In addition, their muscle and bone mass were highly preserved, even at later time points after OA was established. Moreover, control animals presented a higher variability in gait motility in comparison with the Asp-Phe-OMe-fed animals, suggesting a protective effect from movement limitations associated with advanced OA.
Conclusion. Asp-Phe-OMe, given orally, delays OA in the spontaneous STR/ORT model, improves bone cortical density and muscle mass, and may contribute to a better quality of life for these diseased animals.
Keywords: Osteoarthritis, Mice, Bone density, Muscle mass, Gait, Aspartame, Dipeptide aspartyl-phenylalanine-1-methyl ester, Asp-Phe-OMe
Introduction
Over 21 million Americans are estimated to have OA. OA is a chronic, painful, disabling and degenerative disease characterized by ulceration of the cartilage, subchondral bone sclerosis and the presence of osteophytes in the joint. The only available treatment is pain management, which helps to maintain mobility and quality of life. Initiation factors in primary OA are unknown, although genetic susceptibility, mechanical and traumatic injury, endocrine and metabolic factors are thought to play a role [1]. STR/ORT mice are prone to develop spontaneous OA with similar pathophysiology as observed in humans and larger animals [2]. OA can be non-invasively assessed using an established method of radiological scoring [3, 4]; and radiological findings in STR/ORT are comparable with those in human disease, providing a useful tool for exploring therapeutic approaches in OA.
Asp-Phe-OMe, or aspartame, is a U.S. Food and Drug Administration (FDA)-approved food additive for human consumption with a generally recognized as safe (GRAS) designation. To date, aspartame has been demonstrated to be harmless for human consumption [5, 6], and health benefits associated with lower intake of calories reduce a number of illnesses, such as diabetes and cardiovascular diseases [7]. Although aspartame has not been used as medication in any other disease models, there are studies from us and others showing the analgesic effects of aspartame in human OA [8] and in the rodent models of pain [9], also associated with arthritis [10]. The hypothesis of this study was that orally administered aspartame would improve clinical indicators of OA progression such as pain, associated inflammation and bone loss. Therefore, we set out to test the effects of orally administered aspartame vs untreated controls in the spontaneous model of OA in STR/ORT mice.
Our results demonstrate that oral Asp-Phe-OMe significantly delays disease onset in the spontaneous STR/ORT model of OA and improves gait as well as bone and muscle mass at early and late stages of the disease. Asp-Phe-OMe may also enhance clinically relevant aspects for the diseased animals, because gait motility is preserved. These studies reveal a previously unrecognized protective effect of dipeptide aspartame in degenerative osteopathic diseases, and may contribute to an improved quality of life in individuals affected with OA.
Materials and methods
Animals and diets
STR/ORT mice were acquired from National Institutes of Health and bred in the Oklahoma Medical Research Foundation (OMRF) vivarium. All experiments were performed under a protocol approved by the institution’s guidelines for animals’ usage and care. Mice were weaned at the age of 3 weeks, placed in individual cages and randomly classified into Groups A and B. After data collection and analysis, it was revealed that Group B (23 animals, 52% females) received regular mouse chow and Group A (26 animals, 53% females) received the test aspartame-containing diet. The test diet was manufactured at Purina Mills and consisted of chow enriched with aspartame at the concentration of 4 mg/kg. Weight and food consumption were measured weekly for all animals.
In vivo and radiological studies
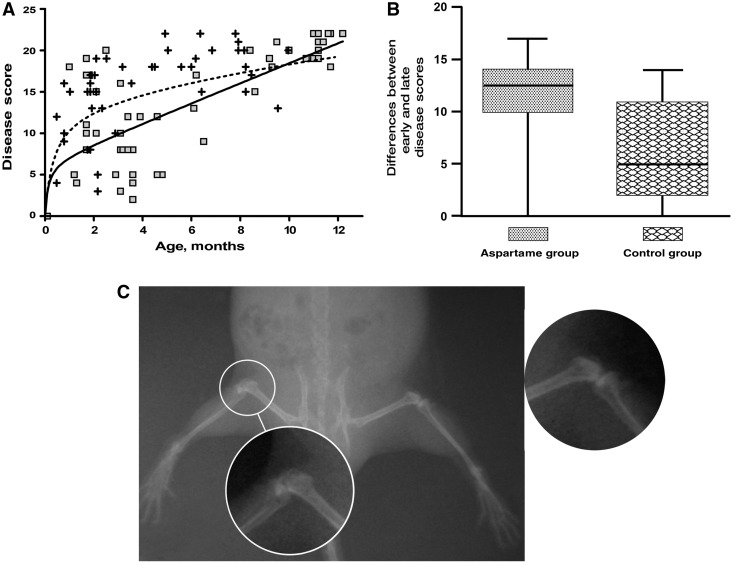
Every 3–5 months, all animals were anesthetized by i.p. injections and submitted to whole-body radiographic scan. Radiographs were copied and transferred to high definition digital archives using animals identification numbers, but without group assignments. Images (jpg archives) were enlarged and compared at similar gamma and transmission densities. All saturations related to colour, film base, light and other sources were digitally eliminated (reduced to zero). Direct measurements of disease abnormalities (radiographic density) were recorded from the digital value of the observed jpg images. Maximal values (digital values 0–255 grey scale) were obtained from the pointer placement on the radiographed abnormality or structure of interest and density demonstrated on the linear gamma curve. Evaluations of all JPEG images were completed by the same experienced technician in a blinded fashion. In addition to direct measurements of transmission, densities from the radiographs in points distant from the femoral head and acetabulum, which were used for the evaluations of bone mass densities, observations for signs of joint damages in each back limb knee were also performed (Fig. 1C). Observations for signs of damaged joints were given a score of 0 if absent, or increasing scores from 1 to 3 according to the various degree of prevalence of radiological abnormalities: including swelling, joint space, irregular articular surface, osteophytes, bone chips, bone deformation, bone resorption and subluxation. The minimal score could be 0 for healthy joints, or varied from 1 to a maximum observed score of 22 in severely affected OA animals. Gait measurements were obtained by standard techniques measuring the ink footprints on the paper as described [11]. Briefly, paws of the test mouse were painted with different colours before placing it in a run/walk, open-ended corridor lined with paper (3 feet in length, 5 inches in width and 5 inches in height). Variability in stride length (distance between adjacent footprints of the same paw) for at least seven legible strides were observed per animal and plotted as average +s.d. per group.
Fig. 1.
Dietary aspartame delays spontaneous OA in STR/ORT mice. (A) Total disease scores for both rear limbs per animal for Asp-Phe-OMe-fed animals (squares) and controls (crosses). Non-linear regression curves for each group (solid line for Asp-Phe-OMe and dashed line for controls) denote a delay in disease for the Asp-Phe-OMe-fed group before the age of 8 months (P-value of 0.0047). (B) Averaged group values of early disease scores deducted from latest disease scores for each animal demonstrate a larger difference for the aspartame-fed group because of delayed disease incidence presenting lower scores within the first 5 months of life (P-value of 0.001). (C) Example of radiographic image of healthy (side enhancement) and OA joint (encircled in white) from one animal.
Statistical analysis
Original data tables were transferred from Excel into GraphPad Prism, which was used for data handling, plotting and statistical analysis. Control and aspartame intergroup differences for disease scores, cortical bone and femoral muscle mass densities, gait and weight were analysed by analysis of variance (ANOVA), linear and nonlinear regressions using GraphPad Prism. Individual comparisons in the analysis satisfied the criteria for t-test. P-values are indicated in the figure legends.
Results
Control and Asp-Phe-OMe-fed STR/ORT animals displayed comparable lifespans and similar variations in food consumption and weight gain
Weight and food consumption were measured weekly in all animals (housed individually) for the duration of the experiments (15 months). No differences were observed for weight variation or food consumption between the Asp-Phe-OMe-fed and the control groups (data not shown).
Spontaneous arthritis is delayed in Asp-Phe-OMe-fed STR/ORT animals in comparison with controls receiving regular diet
Progression of OA can be assessed non-invasively using an established method of radiological scoring [3, 4]. We have used radiological scoring to periodically evaluate the animals fed with the Asp-Phe-OMe diet and regular diet. Radiographic images (exemplified in Fig. 1C) were blindly scored by an expert for radiological signs of joint damage in the major joints of the two hind limbs by the presence of osteophytes, bone deformation or resorption, subluxation and swelling (as described in the ‘Materials and methods’ section). Interestingly, the Asp-Phe-OMe diet significantly delayed disease in the first 10 months of life (Fig. 1A). To demonstrate the differences between early scores per treatment groups more clearly, the first disease score of each animal was deducted from the latest score for the same animal, and the group differences were averaged. The larger differences were clearly observed among Asp-Phe-OMe-treated animals because of the lower scores early on and the delayed disease onset for these animals (Fig. 1B).
Dietary Asp-Phe-OMe maintains normal gait and enhances cortical bone density and muscle mass in the STR/ORT spontaneous murine model of OA
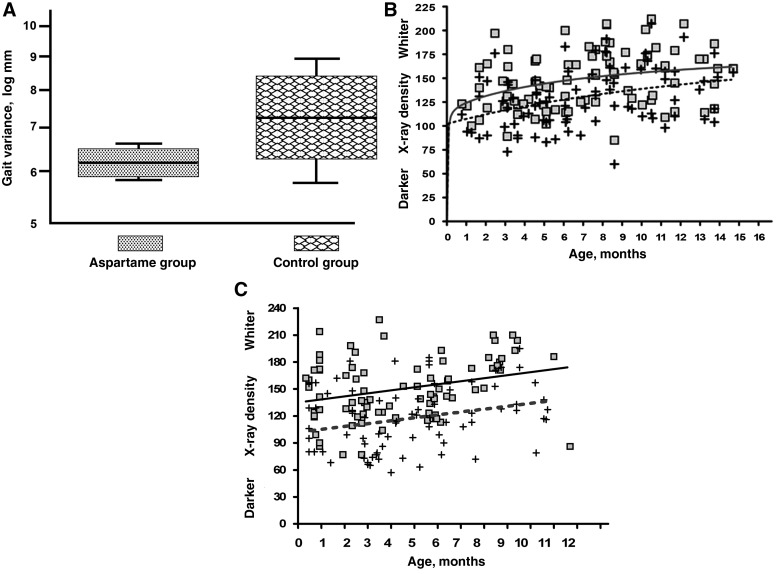
Animals from both groups were tested for mobility by standardized motility gait tests [11] at the age of 8 months to observe if similar joint disease (Fig. 1A) was translated into abnormal gait. Interestingly, control animals demonstrated a more abnormal variable gait in comparison with Asp-Phe-OMe-fed animals (Fig. 2A), which may indicate a more limited movement in the control group probably due to pain. Bone and muscle mass loss are side effects observed in association with OA and could be derived by disuse or limited movement, due to pain and/or cysts or osteophytes in the joint space. Digitized radiographs from all rear limbs were evaluated and scored for bone and muscle mass by an experienced technician in a blinded fashion at different time points. The results demonstrate that femoral cortical bone density was significantly higher from an early age in STR/ORT animals fed Asp-Phe-OMe in comparison with animals fed regular chow (Fig. 2B). The higher bone cortical density was clearly visualized by digitized density measurements, which appeared whiter in the radiographs due to the increased mineral content. Furthermore, mass density of the quadriceps muscle in the hind legs were also found to be preserved in aspartame-fed animals vs controls by digitized radiological evaluations (Fig. 2C). Interestingly, this higher bone and muscle density for aspartame-treated animals was maintained beyond 8 months, despite the significant joint damage observed in both groups (Fig. 1A). These results indicated a protective role of Asp-Phe-OMe in OA-associated bone loss and in the maintenance of muscle mass.
Fig. 2.
Gait, femoral cortical bone and muscle mass are preserved in aspartame-fed animals. (A) Gait variations for aspartame-fed animals and controls. Aspartame-fed and control digitized density values obtained from periodical radiographs of individual animals. (B) Differences in average curves of femoral cortical bone density for the aspartame group (solid line, squares for individual values) and controls (dashed lines, crosses for individual values) are statistically significant (P-value of 0.001). (C) Differences in average curves of femoral muscle mass for aspartame group (solid line) and controls (dashed lines) are statistically significant (P-value of 0.006).
Discussion
Asp-Phe-OMe or aspartame has been used by millions of people for many decades, at tens of milligram levels daily in diet drinks and food. Aspartylphenylalanine, a dipeptide containing l-aspartic acid and l-phenylalanine, is a regular constituent of proteins. Aspartame (the methyl ester of aspartylphenylalanine) undergoes absorption, distribution, metabolism and excretion after in vivo oral administration similar to any other dipeptide or free amino acids. Studies using radiolabelled aspartame or phenylalanine given as a single dose of 20 mg/kg by gavage to rats revealed a similar pattern of tissue distribution and clearance [12]: high levels of radioactivity were observed within 2 h after administration, but values decreased significantly after 24 h. In addition to expected elevated radioactive levels in the stomach and gastrointestinal mucosal tract and its glands, the spinal column and bone marrow also contained significant radioactivity [12].
OA is a progressive, deforming degenerative disease with intercalated episodes of inflammation, occurring spontaneously in rodents, larger mammals and humans. Our interest in testing for protective effects of Asp-Phe-OMe in the murine model of OA derived from previous observation of its beneficial analgesic effects in human OA [8] and the dosage of Asp-Phe OMe chosen for this study was based on these previous studies. Aspartyl-phenylalanine-1-methyl ester was also determined to be an inhibitor of cyclo-oxygenase enzymes, COX-1 (IC50 = 0.1 µM) and COX-2 (IC50 = 0.3 µM) (T. Hugli, unpublished data), thus demonstrating a potent anti-inflammatory function for aspartame, which exhibits many of the analgesic functions of aspirin and other NSAIDS [9]. Since its breakdown product (Asp-Phe) contains two carboxylic acid groups, it should bind divalent cations such as calcium ions, which would increase calcium solubility/bioavailability. Preliminary mass spectral analyses of Asp-Phe (using the + TOF-MS Time-of-flight Mass Spectrometry method) gave a peak at 281 μ. When a 5-fold molar excess of calcium was added, a new peak was detected at 319 μ (281 μ for Asp-Phe + 40 μ for calcium − 2 hydrogen atoms), indicating that the free dipeptide, indeed, binds calcium (T. Hugli, unpublished data). Based on these unpublished observations, a spontaneous OA model using the STR/ORT mice was selected to investigate whether Asp-Phe-OMe could provide any protection against OA.
Asp-Phe-OMe mixed in the animal food diet was well tolerated and no difference in food consumption or weight gain was observed between controls receiving a regular diet or diet enriched with aspartame (data not shown). Asp-Phe-OMe diet did not protect animals from developing OA, but the disease process was significantly delayed in the animals fed Asp-Phe-OMe compared with controls (Fig. 1). We believe that the anti-inflammatory effect of Asp-Phe-OMe contributed to a larger muscle mass (Fig. 2C) due to less pain and better mobility in diseased animals, as tested by gait analysis (Fig. 2A). Painful movements, especially in lower limbs, are associated with diminished muscle mass, which in turn may result in negative (catabolic) bone turnover [13, 14]. Moreover, an unexpected effect of Asp-Phe-OMe was an increase in bone density, suggesting potential therapeutic effects of aspartame in other disease scenarios where decreasing bone mass occurs.
Radiographic evaluations of bone densities are intricate measurements requiring substantial attention to detail. Cortical density measurements appear impacted by radiographic techniques, animal positioning on X-ray plates and consistency in the development of X-ray films. For that reason, radiographs were digitally normalized to the same reference scale for both Asp-Phe-OMe-treated and regular diet animals. Hence, all cortical density radiographic differences observed in controls and aspartame-treated animals were confirmed and remained statistically significant when the ANOVA was performed.
Modelling and remodelling occurring in the subchondral bones are continuously adapting to the pressures and geometry of the joints. In OA patients, the periarticular bone material density is significantly less mineralized due to increased rate of bone turnover, which is accompanied by increasing stiffness of the joint [13, 15]. Therefore, treatments such as the Asp-Phe-OMe, which may help to control the pain (without evidence of the collateral gastric effects of other NSAIDs), could delay the natural evolution of the disease as well as enhance mobility and maintain bone density. Consequently, Asp-Phe-OMe at proper dose levels may provide a better quality of life as a support treatment for patients with advanced OA.
Acknowledgements
The experimental studies reported in this manuscript were performed at Oklahoma Medical Research Institute several years before a company called HealthAide, Inc., was formed. Three of the authors of this manuscript (C.V.M., A.B.E. and T.E.H.) have since become founders of HealthAide, Inc. and, as such, own stock in this company. None of the studies of this manuscript was conducted under either the directions or support from HealthAide, Inc. None of the authors has or will receive any benefit or commercial support from HealthAide, Inc. as a result of this publication. Because HealthAide, Inc. develops products containing the ingredient aspartame, we acknowledge our interest in the subject and in the result of this study. Therefore, we openly report our commercial association with this company as a disclaimer.
Funding: This work was supported by the National Institute of Health (NCI grant CA 72803, to A.B.E.); and by the Oklahoma Medical Research Foundation.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 2.Walton M. Degenerative joint disease in the mouse knee; radiological and morphological observations. J Pathol. 1977;123:97–107. doi: 10.1002/path.1711230206. [DOI] [PubMed] [Google Scholar]
- 3.Evans RG, Collins C, Miller P, Ponsford FM, Elson CJ. Radiological scoring of osteoarthritis progression in STR/ORT mice. Osteoarthr Cartil. 1994;2:103–9. doi: 10.1016/s1063-4584(05)80060-3. [DOI] [PubMed] [Google Scholar]
- 4.Anderson-MacKenzie JM, Billingham ME, Bailey AJ. Collagen remodeling in the anterior cruciate ligament associated with developing spontaneous murine osteoarthritis. Biochem Biophys Res Commun. 1999;258:763–7. doi: 10.1006/bbrc.1999.0713. [DOI] [PubMed] [Google Scholar]
- 5.Butchko HH, Stargel WW, Comer CP, et al. Aspartame: review of safety. Regul Toxicol Pharmacol. 2002;35:S1–93. doi: 10.1006/rtph.2002.1542. [DOI] [PubMed] [Google Scholar]
- 6.Leon AS, Hunninghake DB, Bell C, Rassin DK, Tephly TR. Safety of long-term large doses of aspartame. Arch Intern Med. 1989;149:2318–24. [PubMed] [Google Scholar]
- 7.Renwick AG, Nordmann H. First European conference on aspartame: putting safety and benefits into perspective. Synopsis of presentations and conclusions. Food Chem Toxicol. 2007;45:1308–13. doi: 10.1016/j.fct.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Edmundson AB, Manion CV. Treatment of osteoarthritis with aspartame. Clin Pharmacol Ther. 1998;63:580–93. doi: 10.1016/S0009-9236(98)90109-6. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Jain NK, Kulkarni SK. Possible analgesic and anti-inflammatory interactions of aspartame with opioids and NSAIDs. Indian J Exp Biol. 2005;43:498–502. [PubMed] [Google Scholar]
- 10.LaBuda CJ, Fuchs PN. A comparison of chronic aspartame exposure to aspirin on inflammation, hyperalgesia and open field activity following carrageenan-induced monoarthritis. Life Sci. 2001;69:443–54. doi: 10.1016/s0024-3205(01)01136-5. [DOI] [PubMed] [Google Scholar]
- 11.Lin CH, Tallaksen-Greene S, Chien WM, et al. Neurological abnormalities in a knock-in mouse model of Huntington’s disease. Hum Mol Genet. 2001;10:137–44. doi: 10.1093/hmg/10.2.137. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzawa Y, O’Hara Y. Tissue distribution of orally administered isotopically labelled aspartame in the rat. In: Stegink Lewis D, Filer LJ., editors. Aspartame physiology and biochemistry. NewYork: Marcel Dekker, Inc.; 1984. pp. 161–99. [Google Scholar]
- 13.Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthr Cartil. 2004;12(Suppl. A):S20–30. doi: 10.1016/j.joca.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Turner RT, Lotinun S, Hefferan TE, Morey-Holton E. Disuse in adult male rats attenuates the bone anabolic response to a therapeutic dose of parathyroid hormone. J Appl Physiol. 2006;101:881–6. doi: 10.1152/japplphysiol.01622.2005. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Aspden RM. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J Bone Miner Res. 1997;12:641–51. doi: 10.1359/jbmr.1997.12.4.641. [DOI] [PubMed] [Google Scholar]