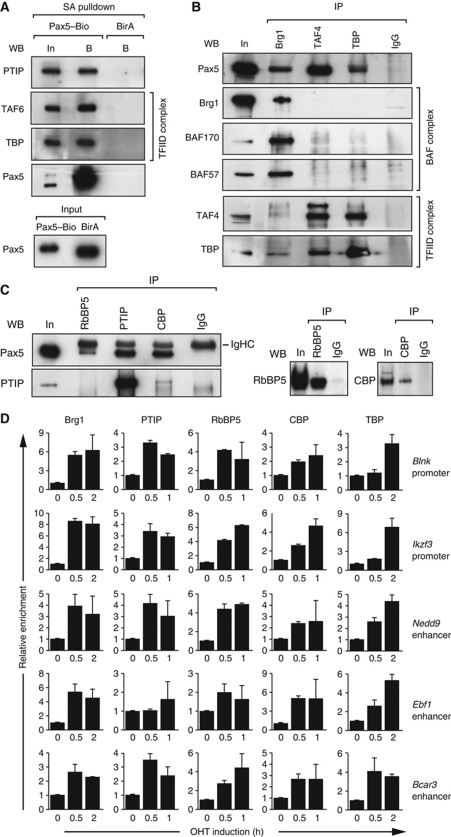
Figure 7.
Pax5 rapidly recruits histone-modifying, chromatin-remodelling and basal transcription factor complexes to activated Pax5 target genes. (A) Co-precipitation of PTIP, TAF6 and TBP with Pax5–Bio. Abelson murine leukaemia virus (Ab-MLV)-transformed pro-B cells of the Pax5Bio/Bio (Pax5–Bio) or control Rosa26BirA/BirA (BirA) genotype were used for streptavidin (SA) pulldown of nuclear extracts. The input (In) fraction (1/100) and streptavidin-bound (B) precipitate were analysed by western blotting (WB) with antibodies (Abs) detecting the indicated proteins. Pax5 was present in similar amounts in both input fractions. (B, C) Co-immunoprecipitation of Pax5 from nuclear extract of Ab-MLV-transformed Pax5Bio/Bio (B) and Rag2–/– (C) pro-B cells with Brg1, TAF4, TBP, RbBP5, PTIP or CBP antibodies. In panel (B), Pax5 was visualized in the immunoprecipitate (IP) by western blotting with a biotinylated rat anti-Pax5 mAb (detected with streptavidin-coupled horse radish peroxidase). Input (In; 1/100) and rabbit IgG were used as controls. Only one tenth of the immunoprecipitated fractions were used for western blotting with the Brg1 antibody. In panel (C), Pax5 was detected with unlabelled rat anti-Pax5 mAb, which was visualized with an anti-rat IgG Ab that crossreacted with the heavy-chain (IgHC) of the rabbit IgG Abs (left). Only one tenth of the immunoprecipitated fractions were used for western blotting with RbBP and CBP antibodies (right). (D) Rapid recruitment of histone-modifying, chromatin-remodelling and basal transcription factor complexes to activated Pax5 target genes. KO-Pax5–ER pro-B cells were treated for up to 2 h with 4-hydroxytamoxifen (OHT, 1 μM) before ChIP with antibodies precipitating the indicated proteins. Input and precipitated DNA were quantified by real-time PCR with primer pairs amplifying the Pax5-binding regions of the indicated genes and the control Tbp promoter. The enrichment of precipitated DNA at the target sites relative to the Tbp promoter was determined as described in the legend of Figure 6A. The relative enrichment at time point 0 was set to 1. The average values and standard deviations of two independent experiments are shown.