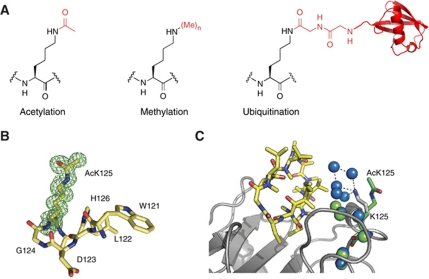
Figure 3.
Genetically encoding lysine acetylation, methylation and ubiquitination. (A) The chemical structures of the post-translationally modified amino acids. (B) Omit map of acetyl lysine density in acetylated cyclophilin A, contoured at 1σ. (C) Structural comparison of the acetylated and unacetylated cyclophilin A–cyclosporine complexes. Cyclosporine is in yellow. The backbone structure of cyclophilin and acetylated cyclophilin are very similar. The backbone of cyclophilin A from a previous structure (PBB 2CPL) is shown in grey and the acetyllysine from our structure is in green. Waters belonging to the unacetylated complex are in blue, waters belonging to the acetylated complex are in green. Acetylation leads to a reorganization of the water network at the interface. This rationalizes why acetylating cyclophilin leads to a 20-fold lower affinity for cyclosporine that may antagonize the immunosuppressive effects of cyclosporine.