Fig. P1.
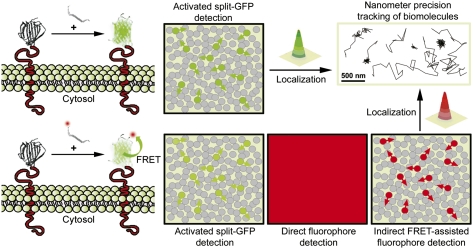
Complementation-activated light microscopy (CALM) imaging. A protein of interest is fused to the large fragment of a nonfluorescent split-GFP, expressed in cells and detected by exogenously providing synthetic versions of the peptide fragment complementary to the split-GFP. The activation of GFP depends on the stochastic binding of the complementary fragment and the chromophore maturation time. Adjusting peptide concentrations and incubation times allows for only a subset of the fusion proteins to be switched on (green circles), despite their high expression in cells (gray circles). Thousands of individual proteins can be progressively highlighted and continuously detected with minimal background. This allows for a high-resolution localization of their position by Gaussian fitting and nanometer precision tracking of their diffusion in living cells. Using split-GFP as Förster resonance energy transfer (FRET) donors and fluorescently labeled complementary peptides, specific targeting and high-resolution tracking of individual fusion proteins was also performed in the low-background far-red region of the light spectrum (red circles) even at fluorophore concentrations that normally prevent SM imaging (direct detection).