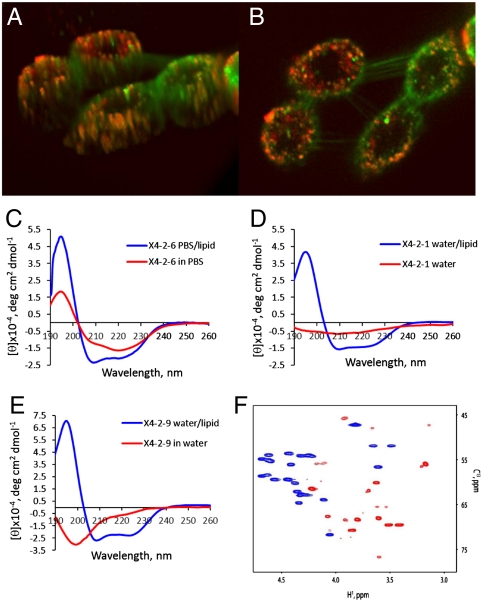
Fig. 2.
Self-assembled particles fuse spontaneously with the cell membrane. Fusion with lipids is accompanied by transition in the peptide conformation from beta-type to helical. (A, B) Confocal laser scanning microscopy images of A549 cells labeled with membrane marker, DiOC18(3) (green) after 20 min treatment with 0.05 mg/mL rhodamine red derivative of X4-2-1 (red). Colocalization of the two appears as yellow or orange. (A) The 3D image of cells exposed to nanoparticles generated from Z-section scanning of the cells. (B) Optical planar section of the cells presented in (A). Peptide appears to saturate intracellular membranes. (C) CD spectra of X4-2-6 micelles demonstrate transition from predominantly beta-type conformation in aqueous solution into an α-helix upon fusion with the lipid. (D) CD spectra of nanoparticles-forming peptide with a tendency to super aggregation, X4-2-1 (D) CD spectra of a peptide that form fibrils, X4-2-9. (F) An overlay of Cα regions of 13C HSQC spectra of X4-2-1 in 100% DMSO - d6 (blue) and in dodecylphocphocholine-d28 micelles (red). A profound downfield shift of 13Cα resonances and an upfield shift of 1Hα resonances indicate that the X4-2-1 peptide adopts a helical conformation in dodecylphosphocholine micelles.