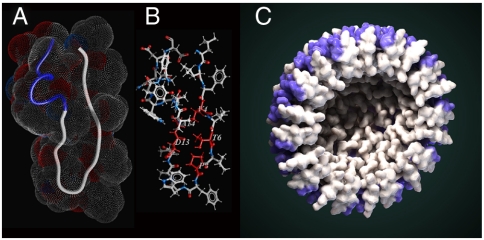
Fig. 3.
Structure of monomeric X4-2-1. (A) Overall fold of X4-2-1 represented as a tube with space-filling topology presented as a mesh. Blue part of the tube corresponds to the C-terminal alpha-helix. (B) The average structure of X4-2-1 showing side-chain positions. The residues stabilizing the hairpin conformation of X4-2-1 are colored red and their types and sequence positions are marked. (C) Hypothetical model of X4-2-1 self-assembly. Cross-section of the nanoparticle is presented. Alpha-helical portion of the peptide is highlighted in blue. Autodesk 3ds Max software was used for the generation of the model.