Abstract
Small molecules of biological origin continue to yield the most promising leads for drug design, but systematic approaches for exploring nature’s cache of structural diversity are lacking. Here, we demonstrate the use of 2D NMR spectroscopy to screen a library of biorationally selected insect metabolite samples for partial structures indicating the presence of new chemical entities. This NMR-spectroscopic survey enabled detection of novel compounds in complex metabolite mixtures without prior fractionation or isolation. Our screen led to discovery and subsequent isolation of two families of tricyclic pyrones in Delphastus catalinae, a tiny ladybird beetle that is employed commercially as a biological pest control agent. The D. catalinae pyrones are based on 23-carbon polyketide chains forming 1,11-dioxo-2,6,10-trioxaanthracene and 4,8-dioxo-1,9,13-trioxaanthracene derivatives, representing ring systems not previously found in nature. This study highlights the utility of 2D NMR-spectroscopic screening for exploring nature’s structure space and suggests that insect metabolomes remain vastly underexplored.
Keywords: chemical ecology, chemical prospecting, metabolomics, natural products, structure elucidation
Discovery of new chemical entities (NCEs) from nature continues to be one of the most prolific methods of finding lead compounds for pharmaceutical and agrochemical development. However, traditional methods for natural products discovery have increasingly led to the isolation and characterization of known compounds, which has reduced enthusiasm for natural products screening programs significantly (1–3). We herein demonstrate accelerated discovery of NCEs based upon (i) biorational selection of taxa for chemical investigation and (ii) assessment of the likelihood of finding NCEs based on 2D NMR-spectroscopic screening of unfractionated extracts.
Arthropods encompass a largely unexplored realm of chemodiversity, even though many arthropod species are abundant and easily collectible, and a vast body of literature exists describing their ecology and taxonomy (4). For this study, we selected a set of 10 insect species (Table 1) whose ecology suggested the presence of potentially interesting natural products. Larvae of all 10 species are coated with hairs bearing oily liquids of previously unknown composition that likely serve defensive functions. Earlier studies had shown that such defensive secretions may harbor genuinely unique types of natural products (5). All 10 species can be obtained in large quantities via collection, culturing, or commercial sources.
Table 1.
Insect species investigated by NMR-spectroscopic screening
| Species, family | Life stage | Source | Diet |
| Delphastus catalinae, Coccinellidae | pupae | laboratory colony* | whiteflies (Bemisia tabaci) raised on either collards (Brassica oleracea) or tobacco (Nicotiana tabacum) devoid of trichome secretion |
| Delphastus catalinae, Coccinellidae | adults | laboratory colony or directly from supplier* | same as above |
| Pieris napi, Pieridae | larvae (2nd or 3rd instar) | laboratory colony† | collards (Brassica oleracea) |
| Pieris virginensis, Pieridae | larvae (2nd to 4th instar) | first-generation progeny of field-collected females‡ | toothwort (Cardamine [Dentaria] spp.) |
| Anthocharis midea, Pieridae | larvae (2nd or 3rd instar) | first-generation progeny of field-collected females§ | rockcress (Arabis spp.) |
| Agraulis vanilla, Nymphalidae | larvae (1st or 2nd instar) | from eggs obtained from suppliers¶ | passion vine (Passiflora caerulae) |
| Megisto cymela, Nymphalidae | larvae (1st instar) | first-generation progeny of field-collected females§ | grass species (Poaceae) |
| Schizura ipomoeae, Notodontidae | larvae (2nd or 3rd instar) | first-generation progeny of field-collected females§ | sugar maple (Acer saccharum) |
| Schizura unicornis, Notodontidae | larvae (2nd or 3rd instar) | first- and second-generation progeny of field-collected females§ | aspen (Populus tremuloides) |
| Schizura leptinoides, Notodontidae | larvae (2nd or 3rd instar) | first-generation progeny of field-collected females§ | shagbark hickory (Carya ovata) |
| Heliothis virescens, Noctuidae | larvae (3rd instar) | laboratory colony∥ | wild-type tobacco (Nicotiana tabacum) with trichome secretion |
*Established from livestock supplied by Applied Bio-nomics, Ltd.
†Progenitors collected in Vermont.
‡Connecticut and Massachusetts.
§Connecticut.
¶Cockerell Butterfly Center, Houston, Texas and Gulf Coast Butterflies, Naples, Florida.
∥Progenitors purchased from the North Carolina State University Insectary.
1H NMR spectra of small-molecule mixtures are frequently used to guide isolation of compounds represented by “unusual” NMR signals (6, 7). However, in much the same way that 1H NMR spectra are unsuitable for the characterization of biological macromolecules such as proteins and nucleic acids, 1H NMR spectra of complex small-molecule mixtures do not provide enough information to assemble well-defined structural fragments suitable for database searches.
Similar to the use of 2D NMR spectroscopy for characterizing biological macromolecules, COSY and other types of 2D spectra have been used for the identification of known compounds from small-molecule mixtures (8, 9). However, detection and characterization of unique or unexpected small molecules in complex mixtures require specific adaptations of the NMR-spectroscopic approach. In previous studies, we introduced high-resolution dqfCOSY spectra of small-molecule mixtures as a means for detecting chemically labile compounds or compounds associated with a specific genetic background (10–12). Here, we employ this strategy to survey the structure space of unfractionated extracts derived from the above set of insect samples. We demonstrate that 2D NMR-based screening for NCEs can be used to generate libraries of structural fragments (“partial structures”) that enable early detection of previously undescribed chemistry. This approach led to the discovery of the catalipyrones (1–10) from the tiny ladybird beetle Delphastus catalinae (Fig. 1). The α-pyrone- and γ-pyrone-bearing 1,11-dioxo-2,6,10-trioxaanthracene and 4,8-dioxo-1,9,13-trioxaanthracene systems of these polyketides are unprecedented from natural sources.
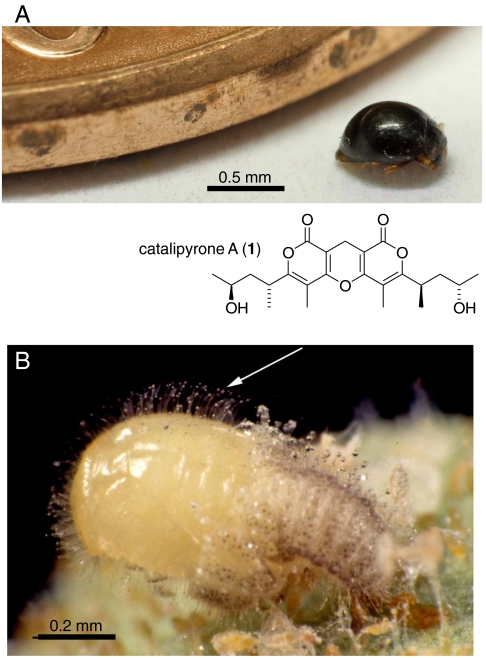
Fig. 1.
Delphastus catalinae. (A) Adult beetle next to rim of a US one cent coin and catalipyrone A (1). (B) Pupa coated with glandular hairs (arrow) emerging from larval skin.
Results
dqfCOSY spectra are particularly suitable for the analysis of complex small-molecule mixtures because they include detailed coupling constant information, often permit clear recognition of long-range 1H, 1H-couplings, and feature easily modeled cross-peak patterns that frequently enable interpretation of overlapping signals (13). As shown in Fig. 2, analysis of dqfCOSY spectra of the 10 insect extracts in this study revealed a large number of partial structures (also see SI Appendix). Four of the 10 extracts were not pursued further because only simple fatty acid derivatives were detected. Analysis of the dqfCOSY spectra of the remaining six samples revealed between 10 and 30 discernible proton spin systems per extract, which for each extract were grouped based on similarity of chemical shifts and coupling constants (Fig. 2).
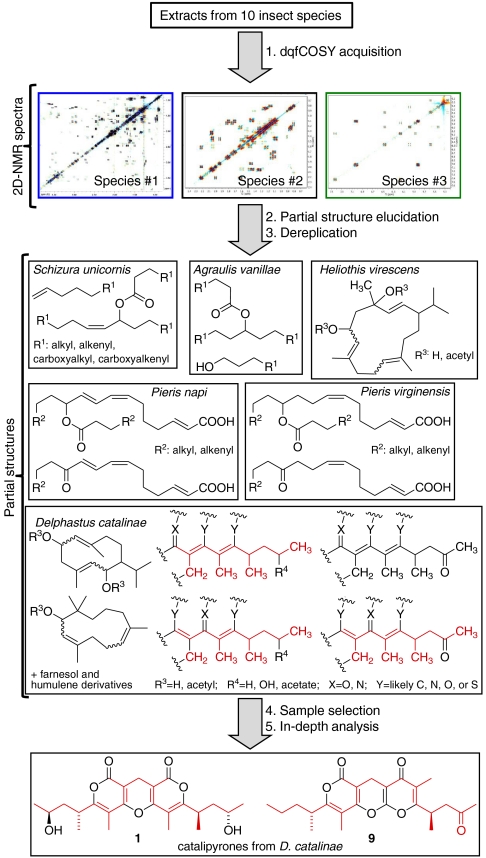
Fig. 2.
Overview of the use of NMR-spectroscopic screening in the discovery of the catalipyrones. Ten largely unfractionated insect metabolite extracts were characterized using 2D NMR spectroscopy, principally high-resolution dqfCOSY spectra. Analysis of the dqfCOSY spectra produced libraries of partial structures. To assess the likelihood of finding NCEs, these partial structures and their NMR-spectroscopic data were compared against available databases. For additional detail on the proposed partial structures, see SI Appendix, Figs. S1–S4 and Table S1.
Spin systems suggestive of structurally more complex metabolites were characterized further using heteronuclear sequential quantum correlation (HSQC) and heteronuclear multiple bond correlation (HMBC) spectra, which enabled constructing the partial structures shown in Fig. 2. Database searches for these partial structures and their NMR-spectroscopic data suggested that the extracts from Schizura unicornis, Agraulis vanillae, and Pieris spp. consist of mixtures of acylated hydroxy fatty acids similar to the mayolenes identified previously from Pieris rapae (5). Similarly, partial structures detected in Heliothis virescens were identified as cembratrienediols and related terpenoids, known constituents of the foliage of Nicotiana tabacum, the host plant of this insect (14). Analysis of the spectra obtained for secretions from pupal Delphastus catalinae revealed partial structures representing sesquiterpenoids, including a humulene derivative and acetylated derivatives of known germacrenediols (15), as well as a large group of polyketide-like fragments, which, based on HMBC data, appeared to be attached to highly unsaturated heterocyclic moieties. Database searches of the polyketide-like fragments and associated NMR-spectroscopic data indicated that these compounds were likely without precedence among known natural products. Comparison with NMR-spectroscopic data of synthetic pyrones further suggested that the Delphastus compounds include polyketide-derived α- or γ- pyrones (16). A portion of this Delphastus dqfCOSY spectrum is shown in Fig. 3A. In this spectrum, the use of high-resolution acquisition parameters (see Materials and Methods) reveals complex cross-peak fine structures that allow distinguishing signals with similar chemical shifts, permit elucidation of coupling constants, and thus provides detailed information on 1H-spin networks, in several cases revealing long-range connectivities (for specific examples, see Fig. S6).
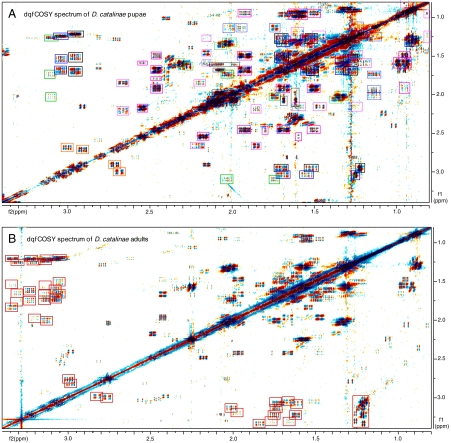
Fig. 3.
Comparison of dqfCOSY spectra obtained for pupal and adult D. catalinae metabolite extracts, illustrating the utility of high-resolution 2D NMR spectra for the comparative analysis of complex metabolite mixtures. (A) 0.8- to 3.4-ppm section of the spectrum obtained for D. catalinae pupae (600 MHz, CD2Cl2), illustrating the level of assignment achieved for this sample. Signals were assigned to different compound classes based on detailed analysis of this dqfCOSY plus HMBC and HSQC spectra. Cross-peaks that could be assigned are boxed; solid pink: germacrene-type sesquiterpenoids; dashed pink: humulene-type sesquiterpenoids; dashed black and blue: farnesol; dashed green: fatty acids. Polyketides: blue (8), orange (6 and isomer), green (10), and black (shared signals of 8–10). (B) Corresponding section of spectrum obtained for D. catalinae adults. The spectrum reveals a large number of polyketide fragments different from those observed in the pupae (1–7, boxed red), in addition to terpenoids and lipids similar to those found in A. See SI Appendix for additional detail.
Based on the detection of partial structures suggesting the presence of unusual polyketide-derived pyrones in Delphastus, we selected this species for further study. Next, we tested whether other life stages of Delphastus produce similar compounds and could provide additional material for analysis. Adult beetles do not have glandular hairs, but dqfCOSY spectra of whole-body extracts of adults revealed large quantities of several compounds with polyketide-like structures related to those detected in the pupal secretion (Fig. 3B). Delphastus adult beetles are used commercially to control whitefly infestations in greenhouses and thus could be obtained in large quantity for isolation and full characterization of the suspected polyketides. Fractionation of adult beetle extracts via preparative HPLC led to isolation of seven different components featuring the polyketide-like partial structures detected in the original screen. For the most abundant component, high-resolution mass spectra indicated a molecular formula of C23H30O7. Because only 12 different carbon signals were observed in this compound’s NMR spectra, the structure had to include two symmetric 11-carbon units. HSQC spectra identified CH2-13 as a methylene unit, and assigning two protons to the integral of the signal from H2-13 in the 1H-spectrum resulted in integrals of two, four, and six protons for all other methine, methylene, and methyl groups, respectively. Therefore, the carbon skeleton of compound 1 appeared to consist of two identical 11-carbon chains attached to the methylene carbon C-13. Subsequently, the presence of the alcohol group at C-3′/C-3″ was established by analysis of 1H NMR data of 1 in protic and aprotic deuterated solvents. At this stage, all carbons and hydrogens were accounted for as were two of the seven oxygens required by the molecular formula. The observed carbon chemical shift values and symmetry requirements determined placement of the remaining five oxygens, yielding a 1,11-dioxo-2,6,10-trioxaanthracene ring system (for additional detail, see SI Appendix). To provide additional evidence for the proposed structure, a 2D INADEQUATE spectrum (17, 18) was acquired using an 18-mg sample of compound 1 obtained from extraction of approximately 25,000 beetles (about 1.5 g). This experiment confirmed all carbon–carbon bonds as originally inferred from the HMBC spectrum of 1.
The 1H NMR spectra of the R- and S-α-methoxy-α-trifluoromethylphenylacetic acid (MTPA) esters of 1 allowed assignment of the absolute configuration at C-3′/C-3″ as S (19). As expected, the spectra of the R- and S-MTPA esters of 1 were different, and only one diastereomer was observed in either case. Significantly, both the R- and S-MTPA esters of 1 showed only one set of 1H NMR signals for the side chains, indicating that the molecule was rotationally symmetrical as opposed to bearing a mirror plane of symmetry (the bis-MTPA derivative of the meso compound would have two sets of signals for the diastereomeric side chains). The absolute configuration at C-1′/C-1″ was determined to be R on the basis of NOESY, molecular modeling, and circular dichroism data (see SI Appendix).
Following the identification of 1, the identification of the other six polyketides isolated from adult Delphastus was straightforward, revealing an additional symmetrical compound (6) as well as several variants with two different side chains, 2–5 and 7 (for complete NMR-spectroscopic data, see SI Appendix). The configurations of compounds 2 and 4–7 were proposed as shown by analogy with 1 based on 1H NMR chemical shift and coupling data and 13C NMR chemical shift data (Fig. 4). Compound 3, however, displayed significantly different NMR-spectroscopic data for the side chain bearing the secondary alcohol (C-1′–C-4′, and C-1′–CH3), indicating inversion of one of the two stereocenters. Based on the observed variability of functionalization at position C-3′ in the Delphastus compounds, we propose that the configuration of C-3′ in compound 3 is R.
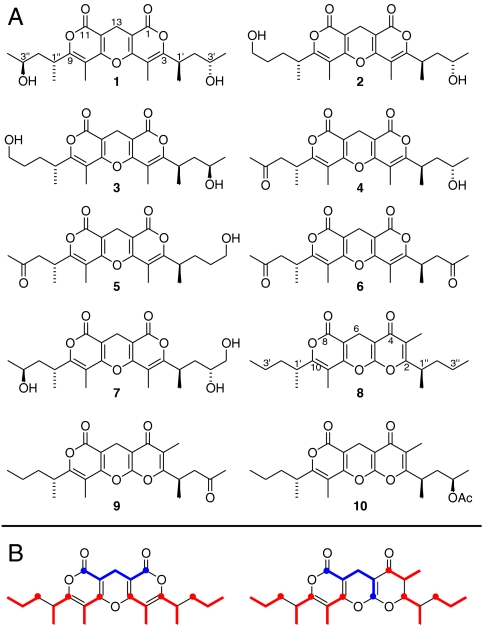
Fig. 4.
Structures of catalipyrones A–J (1–10) from D. catalinae. (A) The 1,11-dioxo-2,6,10-trioxa-anthracenes 1–7 were identified from adult beetles, whereas the 4,8-dioxo-1,9,13-trioxaanthracenes 8–10 are present in the pupal defensive secretion. The stereochemistry of the side chains in 2–9 is proposed in analogy to that determined for 1 (see text). (B) Model for the biosynthesis of 1,11-dioxo-2,6,10-trioxa-anthracenes and 4,8-dioxo-1,9,13-trioxaanthracenes 1–10 from two similarly functionalized tripropionate units (red) and one 5-carbon unit (blue).
Following identification of the identification of 1,11-dioxo-2,6,10-trioxaanthracenes 1–7 in adult D. catalinae, we reanalyzed the dqfCOSY spectra we had obtained for D. catalinae pupae. Comparison of adult and pupal dqfCOSY spectra revealed small differences between the NMR-spectroscopic data of polyketide-like partial structures in the pupal spectra and those of the adult compounds 1–7 (Fig. 3), suggesting that different D. catalinae life stages produce different sets of polyketides. For detailed spectroscopic analysis, we isolated the three major pupal polyketides 8–10 via HPLC. Their HMBC spectra indicated that the pupal compounds 8–10 are based on the same 23-carbon skeleton as adult compounds 1–7; however, correlations to a carbonyl carbon at approximately 180 ppm in 8–10 revealed that these structures are based on the 4,8-dioxo-1,9,13-trioxaanthracene system, instead of the 1,11-dioxo-2,6,10-trioxaanthracene system in 1–7 (see SI Appendix for detailed assignments). The configurations at the chiral centers in 8–10 are proposed to be analogous to those in 1–7, some of which were occasionally found as minor components in the pupal extracts as well (Fig. 4).
Catalipyrones A–J (1–10) from D. catalinae feature two ring systems not previously reported from natural sources. All 10 compounds contain the same 23-carbon skeleton, which could be derived from linear condensation of eight propionyl units. However, the oxygenation pattern as well as the C2 symmetry of the most abundant compound 1 may suggest that the catalipyrones are each derived from condensation of two identically or very similarly functionalized tri- or tetraketides (Fig. 4 and Fig. S19). Intriguingly, simple 4H pyrones based on similarly symmetric polyketide carbon skeletons and oxygenation patterns have been identified from marine sources (20). These structural similarities may hint at possible production by related endosymbionts (21).
For biological evaluation of the D. catalinae pyrones, 10-mg samples of 1–3 were submitted to the National Institutes of Health’s molecular libraries small-molecule repository. In addition, a small portion of the isolated sample of 1 was tested for insect-repellant activity because D. catalinae appears to use these compounds in a defensive context. These assays showed that 1 was more potent in repelling the native ant predator Crematogaster lineolata than a well-known insect repellant, the alkaloid nicotine. Thus D. catalinae is the only known ladybird beetle whose chemical defenses do not appear to be based on nitrogenous compounds (4, 22). D. catalinae pupae and adults appear to use the catalipyrones defensively in two different manners, as pupae secrete a mixture of the less polar catalipyrones 8–10, sesquiterpenoids and other compounds via dedicated glandular hairs, whereas adults beetles expose predators to bodily fluids containing the more polar 1–7 through reflex bleeding, a defense response widespread among ladybird beetles (22). Several of the other insect species examined produce compounds that, though not representing unique types of natural products, may also serve interesting ecological roles.
Discussion
This study demonstrates the utility of 2D NMR-spectroscopic screening for the discovery of previously undescribed natural products and shows that unique chemistry can be found even in seemingly mundane organisms such as D. catalinae, a ladybird beetle species widely used as a biological pest control agent. Similar to MS-based proteomics providing partial coverage of peptide sequences in complex protein mixtures (23), 2D NMR-based screening identifies partial structures in complex metabolite samples, enabling their evaluation based on analysis of small pilot-scale samples. The technique effectively complements analysis of molecular composition data obtained via MS, greatly reducing the likelihood of wasting resources for isolation of known or otherwise unoriginal compounds. It should be noted that using dqfCOSY spectra for NMR-spectroscopic screening provided useful structural data even for minor components representing less than 1% of the total extract (see Figs. S7 and S8), except for cases of severe signal overlap. The method can thus be regarded as a useful alternative to chromatography-based approaches to NCE discovery, such as HPLC-NMR (24). Eliminating or reducing the need for chromatography as the first step in the analysis of biological samples of unknown composition is often desirable, as chromatographic fractionation necessarily risks loss or degradation of components with unexpected chemical properties (25). Use of higher field strength NMR spectrometers than employed in this study (600 MHz) could reduce signal overlap and may further improve detection of minor components. Increased spectral dispersion could also be achieved via heteronuclear 2D or 3D NMR-spectroscopic techniques (26, 27); however, the inherently lower sensitivity of heteronuclear experiments may reduce the ability to detect minor components, thus defeating any improvement derived from reduced signal overlap.
One principal challenge consists in finding ways to fully access the enormous amount of information contained in high-resolution 2D NMR spectra of complex mixtures. In particular, better computational methods (28) will be needed to aid with spectral analysis and deconvolution, in order to further increase both throughput and level of assignment in the spectra.
We believe that when combined with careful selection of organisms for study, nondiscriminatory analyses of natural products mixtures via NMR-spectroscopic screening will make an important contribution to NCE discovery. In addition, the possibility of using 2D NMR-based analyses of mixtures in synthetic organic chemistry, for example for the development of combinatorial methods, seems intriguing.
Materials and Methods
Instrumentation.
NMR spectra were recorded on a Varian INOVA 600 NMR (600 MHz for 1H, 151 MHz for 13C) using a HCN indirect-detection probe, except for direct-detected 13C and 2D-INADEQUATE spectra, which were recorded on a Varian INOVA 500-MHz NMR spectrometer fitted with a DBG probe. NMR spectra were processed using Varian VNMR and MestreLabs MestReC software packages. HPLC-MS was performed using an Agilent 1100 Series HPLC system equipped with a diode array detector and connected to a Quattro II spectrometer (Micromass/Waters). Data acquisition and processing for the MS were controlled by the MassLynx software. High-resolution mass spectrometry was performed on a LTQ Orbitrap Velos (Thermo Scientific). Flash chromatography was performed using a Teledyne ISCO CombiFlash system. Optical rotations were measured in methanol using a Perkin-Elmer model 241 polarimeter.
Insect Materials.
For each insect species examined (Table 1), samples were obtained by collective placement of specimens in dry-ice chilled vials. These were stored at -80 °C until shipment on dry ice to Cornell.
Collection of Insect Metabolite Samples.
For collection of surface-wash samples, insect specimens, pipettes, and dichloromethane were precooled to -78 °C in dry ice. The insect samples (about 100-mg frozen specimens) were rinsed twice with 1-mL cold dichloromethane. The resulting extracts were filtered evaporated to dryness in vacuo at 20 °C. Whole-body D. catalinae extracts were prepared as follows. Adult D. catalinae were pulverized in a mortar and pestle and extracted three times with ethyl acetate to afford a crude extract. For the pilot-scale extraction approximately 500 beetles were used (counted). For the scaled-up extraction, approximately 25,000 beetles were used (by weight).
NMR-Spectroscopic Characterization of Insect Metabolite Samples.
Metabolite samples that were entirely soluble in methanol or chloroform were dissolved in CD3OD or CDCl3, respectively, and analyzed by NMR spectroscopy. Extracts that did not dissolve completely in either of these two solvents (for example, the D. catalinae whole-body extracts) were treated with chloroform, filtered, and the filter residue dissolved in methanol. The chloroform- and methanol-soluble fractions were then analyzed separately. High-resolution dqfCOSY spectra were acquired using the following parameters: 0.6-s acquisition time, 64 increments per ppm sweep width, and 8, 16, or 32 scans per increment and 250–600 complex increments in F1. The numbers of increments were chosen so that the digital resolution in F1 was roughly the same for all extracts. Generally, 40–80 complex increments per 1 ppm of sweep width in F1 were acquired. Additional HMBC and HSQC spectra were acquired for the unfractionated Delphastus catalinae, Pieris napi, Pieris virginensis, Schizura unicornis, and Heliothis virescens samples.
Isolation of Catalipyrones.
Fresh adult D. catalinae ethyl acetate extracts were partitioned between equal volumes of hexanes and acetonitrile to remove excess lipids (surface washes of immature D. catalinae were not partitioned). The acetonitrile layer was then submitted to purification via HPLC on an Agilent series 1100 HPLC system with autosampler, using an Agilent Zorbax-XDB (C-18, 5 μ, 9.4 × 250 mm) column and a constant flow rate of 3.4 mL/ min. A binary solvent system of acetonitrile and water was used starting with 37% acetonitrile, followed by a linear gradient to 52% acetonitrile over 20 min, followed by a steeper linear gradient to 100% acetonitrile over 4 min. The retention times for compounds 1–7 were 9.2 min, 8.7 min, 8.9 min, 12.5 min, 12.1 min, 12.8 min, and 5.1 min, respectively. The HPLC solvent system used to isolate pupal compounds started with 70% acetonitrile in water for 5 min, followed by a linear gradient to 85% acetonitrile over 30 min, followed by 10 min at 85% acetonitrile in water. Retention times for compounds 8–10 were 41.3 min, 16.1 min, and 26.0 min, respectively.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grant GM079571 (to F.C.S.) and by the Eppley Foundation for Research, Howard Hughes Medical Institute, and the Trinity College Faculty Research Committee (S.R.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107020108/-/DCSupplemental.
References
- 1.Ortholand JY, Ganesan A. Natural products and combinatorial chemistry: Back to the future. Curr Opin Chem Biol. 2004;8:271–280. doi: 10.1016/j.cbpa.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 3.Rapaka RS, et al. Drug Addiction. New York: Springer; 2008. Drug discovery from natural sources; pp. 17–39. [Google Scholar]
- 4.Gronquist M, Schroeder FC, Lew M, Hung-Wen L. Comprehensive Natural Products II. Oxford, UK: Elsevier; 2010. Insect natural products; pp. 67–108. [Google Scholar]
- 5.Smedley SR, et al. Mayolenes: Labile defensive lipids from the glandular hairs of a caterpillar (Pieris rapae) Proc Natl Acad Sci USA. 2002;99:6822–6827. doi: 10.1073/pnas.102165699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molinski TF. Nanomole-scale natural products discovery. Curr Opin Drug Discov Devel. 2009;12:197–206. [PubMed] [Google Scholar]
- 7.Molinski TF. NMR of natural products at the ‘nanomole-scale’. Nat Prod Rep. 2010;27:321–329. doi: 10.1039/b920545b. [DOI] [PubMed] [Google Scholar]
- 8.Wei F, Furihata K, Hu F, Miyakawa T, Tanokura M. Complex mixture analysis of organic compounds in green coffee bean extract by two-dimensional NMR spectroscopy. Magn Reson Chem. 2010;48:857–865. doi: 10.1002/mrc.2678. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F, Bruschweiler R. Robust deconvolution of complex mixtures by covariance TOCSY spectroscopy. Angew Chem Int Ed Engl. 2007;46:2639–2642. doi: 10.1002/anie.200604599. [DOI] [PubMed] [Google Scholar]
- 10.Taggi AE, Meinwald J, Schroeder FC. A new approach to natural products discovery exemplified by the identification of sulfated nucleosides in spider venom. J Am Chem Soc. 2004;126:10364–10369. doi: 10.1021/ja047416n. [DOI] [PubMed] [Google Scholar]
- 11.Gronquist M, Meinwald J, Eisner T, Schroeder FC. Exploring uncharted terrain in nature’s structure space using capillary NMR spectroscopy: 13 steroids from 50 fireflies. J Am Chem Soc. 2005;127:10810–10811. doi: 10.1021/ja053617v. [DOI] [PubMed] [Google Scholar]
- 12.Pungaliya C, et al. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2009;106:7708–7713. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edison AS, Schroeder FC, Lew M, Hung-Wen L. Comprehensive Natural Products II. Oxford, UK: Elsevier; 2010. NMR—Small molecules and analysis of complex mixtures; pp. 169–196. [Google Scholar]
- 14.Severson RF, et al. Quantitation of the major cuticular components from green leaf of different tobacco types. J Agric Food Chem. 1984;32:566–570. [Google Scholar]
- 15.Barrero AF, Herrador MM, Arteaga P. Sesquiterpenes and phenylpropanoids from Seseli vayredanum. Phytochemistry. 1992;31:203–207. [Google Scholar]
- 16.Moreno-Mañas M, Ribas J, Sánchez-Ferrando F, Virgili A. 3,6,9-trioxanthracenes and 3,6-dioxa-9-thianthracenes: Conformational analysis and complete assignment of 13C NMR spectra. Magn Reson Chem. 1987;26:625–642. [Google Scholar]
- 17.Buddrus J, Bauer H. Direct identification of the carbon skeleton of organic compounds using double quantum coherence 13C-NMR spectroscopy. The INADEQUATE pulse sequence. Angew Chem Int Ed Engl. 1987;26:625–642. [Google Scholar]
- 18.Bringmann G, et al. Polyketide folding in higher plants: Biosynthesis of the phenylanthraquinone knipholone. J Org Chem. 2007;72:3247–3252. doi: 10.1021/jo062566x. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani I, Kusumi T, Kashman Y, Kakisawa H. High-Field FT NMR application of Mosher’s method the absolute configurations of marine terpenoids. J Am Chem Soc. 1991;113:4092–4096. [Google Scholar]
- 20.Manker DC, Faulkner DJ. Vallartanones A and B, polypropionate metabolites of Siphonaria maura from Mexico. J Org Chem. 1989;54:5374–5377. [Google Scholar]
- 21.Piel J. Metabolites from symbiotic bacteria. Nat Prod Rep. 2009;26(3):338–362. doi: 10.1039/b703499g. [DOI] [PubMed] [Google Scholar]
- 22.Glisan King A, Meinwald J. Review of the defensive chemistry of coccinellids. Chem Rev. 1996;96:1105–1122. doi: 10.1021/cr950242v. [DOI] [PubMed] [Google Scholar]
- 23.Mallick P, Kuster B. Proteomics: A pragmatic perspective. Nat Biotechnol. 2010;28:695–709. doi: 10.1038/nbt.1658. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y, Schiavo S, Orjala J, Vouros P, Kautz R. Microscale LC-MS-NMR platform applied to the identification of active cyanobacterial metabolites. Anal Chem. 2008;80(21):8045–8054. doi: 10.1021/ac801049k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder FC, et al. NMR-spectroscopic screening of spider venom reveals sulfated nucleosides as major components for the brown recluse and related species. Proc Natl Acad Sci USA. 2008;105(38):14283–14287. doi: 10.1073/pnas.0806840105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedenstrom M, et al. Identification of lignin and polysaccharide modifications in Populus wood by chemometric analysis of 2D NMR spectra from dissolved cell walls. Mol Plant. 2009;2(5):933–942. doi: 10.1093/mp/ssp047. [DOI] [PubMed] [Google Scholar]
- 27.Cui Q, et al. Metabolite identification via the Madison Metabolomics Consortium Database. Nat Biotechnol. 2008;26(2):162–164. doi: 10.1038/nbt0208-162. [DOI] [PubMed] [Google Scholar]
- 28.Robinette SL, et al. Hierarchical alignment and full resolution pattern recognition of 2D NMR Spectra: Application to nematode chemical ecology. Anal Chem. 2011;83(5):1649–1657. doi: 10.1021/ac102724x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.