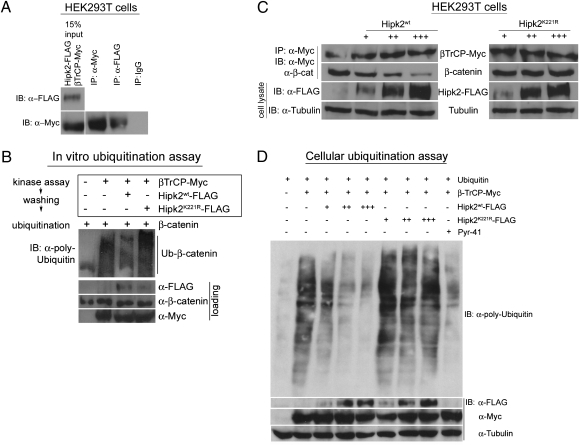
Fig. 3.
Vertebrate Hipk2 displays functional conservation with its Drosophila counterpart and inhibits β-catenin ubiquitination. (A) In a coimmunoprecipitation assay in HEK293T cells, Hipk2 forms a complex with β-TrCP, the substrate recognition domain of the E3 ligase complex. IgG was used as a negative control in the assay. (B) In an in vitro ubiquitination assay, β-catenin is ubiquitinated in the presence of β-TrCP (lane 2) but not in its absence (lane 1). Preincubation of β-TrCP in a kinase assay with Hipk2WT (lane 3) but not Hipk2K221R (lane 4) reduces the β-TrCP–mediated ubiquitination of β-catenin. (C) In HEK293T cells, an increasing dosage series of Hipk2WT proportionately reduces the amount of β-catenin bound to β-TrCP. This effect is kinase-dependent, because kinase-inactive Hipk2K221R has no effect on the amount of β-catenin bound to β-TrCP. (D) HEK293T cells transfected with β-TrCP results in the ubiquitination of multiple cellular substrates. The introduction of increasing amounts of Hipk2WT in the presence of β-TrCP leads to a gradual decline in levels of ubiquitinated cellular proteins, an effect similar to that produced by treating the cells with the E1 inhibitor PYR41. This ability of Hipk2 to inhibit the β-TrCP-mediated ubiquitination is kinase-dependent, because an increasing dosage series of Hipk2K221R has no effect on cellular ubiquitination levels.