Abstract
Background
The debilitating effects of chronic glucocorticoids excess are well-known, but comparatively little is understood about the role of acute cortisol. Indirect evidence in rodents suggests that acute cortisone could selectively increase some forms of long-duration aversive states, such as “anxiety,” but not relatively similar, briefer aversive states, such as “fear.” However, no prior experimental studies in humans consider the unique effects of cortisol on anxiety and fear, using well-validated methods for eliciting these two similar but dissociable aversive states. The current study examines these effects, as instantiated with short- and long-duration threats.
Methods
Healthy volunteers (n = 18) received placebo or a low (20 mg) or a high (60 mg) dose of hydrocortisone in a double-blind crossover design. Subjects were exposed repeatedly to three 150-sec duration conditions: no shock; predictable shocks, in which shocks were signaled by a short-duration threat cue; and unpredictable shocks. Aversive states were indexed by acoustic startle. Fear was operationally defined as the increase in startle reactivity during the threat cue in the predictable condition (fear-potentiated startle). Anxiety was operationally defined as the increase in baseline startle from the no shock to the two threat conditions (anxiety-potentiated startle).
Results
Hydrocortisone affected neither baseline nor short-duration, fear-potentiated startle but increased long-duration anxiety-potentiated startle.
Conclusions
These results suggest that hydrocortisone administration in humans selectively increases anxiety but not fear. Possible mechanisms implicated are discussed in light of prior data in rodents. Specifically, hydrocortisone might increase anxiety via sensitization of corticotrophin-releasing hormones in the bed nucleus of the stria terminalis.
Keywords: Amygdala, anxiety, BNST, corticotropin-releasing hormone (CRH), cortisol, fear, predictability, startle reflex
Understanding the behavioral effects of glucocorticoids has long been of paramount clinical importance, given their role on the stress response and their potential debilitating effects on brain function. Aversive and stressful events release cortisol in humans (corticosterone in rodents) and evoke anxiety. Acutely, cortisol restores homeostasis and enhances emotional memory in both humans and rodents (reviewed in Lupien et al. [1]). Less is known, however, about other psychological effects of cortisol in humans, specifically on aspects of emotional responding. A key question is whether acute cortisol increases or decreases the emotional response of humans to threat. This question is complicated, because aversive states such as fear and anxiety are functionally heterogeneous, reflecting involvement of distinct underlying neural and psychopharmacology mechanisms. In the present study, we argue for a functional differentiation between fear and anxiety and test the hypothesis that acute cortisol affects the latter but not the former.
Prolonged exposure to glucocorticoids increases defensive responses in rodents (2,3), but few studies have focused on the emotional effect of acute cortisol administration (4,5). Acute glucocorticoids have been associated with both increased and decreased defensive responses in animals (3,4,6) and in humans (7–10). These contradictory findings are not surprising given that glucocorticoids exert differential, often poorly understood effects, on several brain regions.
Glucocorticoids could affect anxiety via action on corticotropin-releasing hormone (CRH), which plays a pivotal role in stress and anxiety. Glucocorticoids could relieve anxiety through negative feedback on CRH released from the paraventricular nucleus of the hypothalamus within the hypothalamic-pituitary-adrenal axis, reestablishing homeostasis. Alternatively, glucocorticoids could affect CRH in limbic areas (4,11,12), where CRH receptors affect anxiety independently of hypothalamic-pituitary-adrenal axis (13). In limbic structures, glucocorticoids sensitize rather than inhibit CRH activity (reviewed in Schulkin et al. [14]). Glucocorticoid upregulation of CRH messenger RNA expression has been documented in the central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST) (4,6,11,15,16), structures that have been associated with fear and anxiety, respectively (see following text). In fact, not only chronic but also acute corticosterone can sensitize aversive states in these structures (2,4,16), suggesting that glucocorticoids can enhance aversive states via action on limbic CRH.
What is the possible role of glucocorticoids and their regulation of CRH on fear and anxiety? Strong evidence now indicates that fear and anxiety (operationally defined as aversive responses to short-and long-duration threats, respectively) involve distinct brain regions (reviewed in Davis [17] and Grillon et al. [18]), possibly the CeA and BNST (17). Specifically, phasic fear-potentiated startle to a signaled shock is mediated by the medial division of the CeA, whereas sustained forms of potentiated startle reflex (anxiety-potentiated startle) to threatening contexts are mediated by projections from the basolateral amygdala and lateral CeA to the BNST (17). Importantly, current models further indicate that CRH increases anxiety-potentiated startle but does not affect fear-potentiated startle (19). Indeed, infusion of CRH antagonist into the BNST blocks anxiety-potentiated startle but leaves fear-potentiated startle unchanged, whereas infusion in the CeA influences neither response (reviewed in Davis [17]). These findings together with evidence of corticosterone-mediated upregulation of CRH in the BNST (4,11,14,20) led us to predict that cortisol would increase sustained anxiety states.
This hypothesis was tested in humans by studying the effect of acute hydrocortisone pretreatment on fear to an explicit threat cue and on anxiety to threatening contexts. Explicit threat cues refer to threat signals that predict a shock, whereas threatening contexts refer to conditions where shocks are administered. In rodents, the context usually refers to a spatial location (e.g., the cage), but it can also be a long-duration unimodal stimulus (21). In humans, a context can be a virtual space (22) or the sustained presentation of an ambient light or screen color (23,24). Explicit threat cues evoke a phasic fear response, because the associated threat is imminent and of short-duration, whereas threatening contexts elicit sustained anxious responses. Furthermore, greater anxiety is observed in contexts associated with unpredictable compared with predictable shocks (22,25). We have developed a startle procedure to examine short- and long-duration potentiated startle in response to explicit threat cues (fear-potentiated startle) or aversive contexts (anxiety-potentiated startle), respectively (26). Specifically, subjects are presented with three conditions, no shock (N), predictable shocks (P), and unpredictable shocks (U). In P, shocks are signaled by a short-duration threat cue, whereas shocks are delivered at any time in U. A predictable shock evokes a robust increase in startle reactivity during the explicit threat cue (fear-potentiated startle). Both P and U elicit sustained levels of startle potentiation (anxiety-potentiated startle), relative to N, with greater startle potentiation during U compared with P (22,25). As a result, “baseline” startle reactivity increases linearly from N to P to U (22,25). Clinical and psychopharmacological studies relying on this procedure indicate that fear-potentiated startle and anxiety-potentiated startle reflect functionally distinct aversive states (27–29). Specifically, anxiety-potentiated startle but not fear-potentiated startle is reduced by anxiolytics (alprazolam and citalopram) (29,30) and is increased in panic disorder and post-traumatic stress disorder (27,28). We expected hydrocortisone to enhance anxiety-potentiated startle but not fear-potentiated startle, on the basis of the assumption that cortisol potentiates CRH and that CRH acts only on sustained potentiated startle response.
Methods and Materials
Participants
Participants were paid healthy volunteers who gave written informed consent approved by the National Institute of Mental Health Human Investigation Review Board. Inclusion criteria included: 1) no past or current psychiatric disorders as per Structured Clinical Interview for DSM-IV (31), 2) no history of a psychiatric disorder in any first-degree relatives, 3) no medical condition that interfered with the objectives of the study as established by a physician, and 4) no use of illicit drugs or psychoactive medications as per history and confirmed by a negative urine screen. Participants met with a psychiatrist before providing consent. Twenty-four subjects participated in the study, but 2 did not complete the second session. The final group consisted of 22 subjects (15 male subjects) with a mean age of 27.1 years (SD = 4.3 years).
Drugs
A double-blind crossover design was implemented, with each subject being exposed to each treatment—placebo, 20 mg hydrocortisone, and 60 mg of hydrocortisone— on separate sessions. The order of treatment was counterbalanced across subjects. The treatments were given as identical-appearing capsules 1 hour before testing.
Procedure
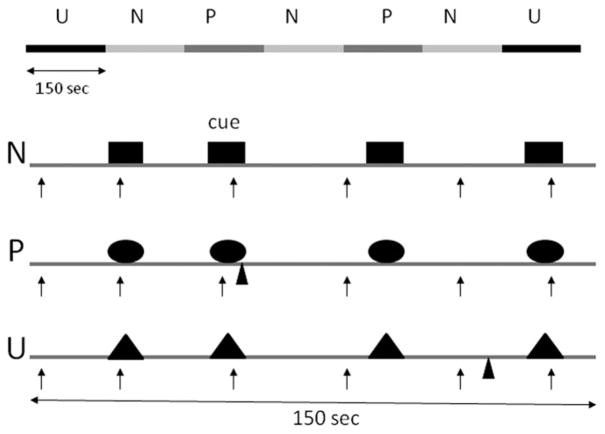
The procedure was similar to that of our previous psychopharmacology studies examining responses to predictable and unpredictable shocks (26–28). Subjects participated in three identical testing sessions separated by 6–9 days. Subjects arrived at 8:30 AM in the laboratory (see Table 1 for timeline). Sixty minutes later, nine startle stimuli (habituation) were delivered every 18–25 sec to reduce initial startle reactivity. Afterward a shock workup procedure was initiated to set up the shock intensity at a highly annoying level. Next, subjects ingested a capsule containing one of the drugs. One hour later, the threat experiment was started. It consisted of three 150-sec conditions (Figure 1), a no shock condition (N), and two conditions during which shocks were administered either predictably (P) (i.e., only in the presence of a threat cue) or unpredictably (U). In each condition, an 8-sec cue was presented four times. The cues consisted of differently colored geometric shapes for the different conditions (e.g., blue square for N, red circle for P, green triangle in U). The cues signaled a shock only in the P condition; they had no signal value in the N and U conditions.
Table 1.
Procedure: Timeline
| Time (min) | Events |
|---|---|
| 0 | Subjects’ arrival |
| 5 | Spielberger state anxiety 1 |
| 45 | Salivary Sample 1 |
| 55 | Salivary Sample 2 |
| 60 | Nine startle (habituation) |
| 70 | Shock work-up |
| 80 | Drug ingestion |
| 130 | Spielberger state anxiety 2 |
| 140 | Threat/series 1 |
| 160 | Retrospective anxiety rating 1 |
| Spielberger state anxiety 3 | |
| Salivary sample 3 | |
| 170 | Threat/series 1 |
| 190 | Retrospective anxiety rating 2 |
| Spielberger state anxiety 4 | |
| Salivary sample 4 | |
| 215 | Salivary sample 5 |
Figure 1.
Schematic of the experiment. There were three conditions: no shock (N), predictable shock (P), and unpredictable shock (U). Each subject was presented with two series, each including three N, two P, and two U in each of the two orders (UNPNPNU as shown or PNUNUNP). Each N, P, and U condition contained four 8-sec cues of different colors and geometric shapes (for illustration purposes, the cues are squares in N, circles in P, and triangles in U). In each P condition, a shock (indicated by ▲) was randomly associated with one of the four threat cues; it was administered 7.5 sec after its onset. In each U condition, a shock was administered randomly in the absence of the cues. In the N condition, no shock was administered. Startle stimuli (indicated by ↑) were delivered in the presence and in the absence of the cue (i.e., during intertrial intervals).
Participants received precise instructions with regard to risk of shock in each condition, including the contingency between shocks and cues in P and U. To minimize involvement of memory processes (which can be affected by cortisol), instructions were also shown on a computer monitor throughout the experiment displaying the following information: “no shock” (N), “shock only during shape” (P), or “shock at any time” (U). In each N, P, and U condition, six acoustic startle stimuli were delivered: 1) three during intertrial intervals (ITI) (i.e., in the absence of cues), one at 15–52 sec, a second at 53–96 sec, and a third at 97–140 sec after the beginning of a condition; and 2) one during three of the four cues, 5–7 sec after cue onset.
The threat experiment consisted of two series with a 5–10 min rest between series. Each series started with the delivery of four startle stimuli (prethreat startle) and consisted of three N, two P, and two U in one of the following two orders: P N U N U N P or U N P N P N U. Each participant received both orders, with one-half of the participants starting with P and the other one-half starting with U. One shock was administered in each individual P and U condition for a total of four shocks in P and four shocks in U. In each P, the shock was randomly associated with one of the four threat cues, being administered 7.5 sec after the onset of that cue. The shock was given either 7 or 10 sec after the termination of a cue in the unpredictable condition. No startle stimuli followed a shock by < 10 sec.
The Spielberger state portion of the State–Trait Anxiety Inventory questionnaire (32) was administered four times: 1) just after arrival of the subjects (predrug), 2) before the first threat series (postdrug), 3) after the first threat series, and 4) just after the second threat series. In addition, after each series, subjects retrospectively rated their anxiety level in the presence and absence of the cue in each condition (N, P, U) on an analogue scale ranging from 0 (not at all anxious) to 10 (extremely anxious). Immediately after the last recording, subjects were also asked to retrospectively rate the level of shock pain experienced during testing on an analogue scale ranging from 0 (not at all painful) to 10 (extremely painful).
Five salivary samples were taken to assess cortisol changes during testing at the following times (Table 1): 1) 45 min and 2) 55 min after arrival of the subjects (just before the startle habituation test), 3) just after the first threat period, 4) just after the second threat period, and finally 5) 25 min later.
Stimuli and Physiological Responses
Stimulation and recording were controlled by a commercial system (Contact Precision Instruments, London, England). The acoustic startle stimulus was a 40-msec, 103-dB burst of white noise presented through headphones. The eyeblink reflex was recorded with electrodes placed under the left eye. The electromyographic signal was amplified with bandwidth set to 30–500 Hz and digitized at a rate of 1000 Hz. The shock was administered on the left wrist.
Salivary Cortisol
Saliva was collected in non-coated Salivettes (Sarstedt, Nümbrecht, Germany) with an absorbent swab placed under the tongue or between cheek and teeth for 2 min. After centrifugation (10 min, 2500 rpm) within 30 min after sampling, the saliva was stored at 20° C until analysis. The concentration of salivary cortisol was measured by a solid-phase radioimmunoassay with a commercially available Coat-A Count Cortisol RIA kit (Siemens Medical Solutions Diagnostics, Los Angeles, California) used as instructed. The inter- and intra-assay coefficients of variation were below 10%.
Data Analysis
The electromyographic eyeblink was rectified and smoothed with a 20-point moving average. Peak amplitude of the startle/blink reflex was determined in the 20–100-msec time frame after stimulus onset relative to a 50-msec pre-stimulus baseline and averaged within each condition, after which they were standardized into T scores on the basis of data across sessions within each participant. Both the T scores and raw-scores are shown in Table 2. Fear-potentiated startle was defined as the increase in startle magnitudes from ITI to the threat cue in the P condition. Anxiety-potentiated startle was defined as the increase in ITI startle reactivity from N to P and from N to U. Data were entered into analyses of variance (ANOVAs) with repeated measures. Alpha was set at .05 for all statistical tests. Greenhouse-Geisser corrections (GG-ε) were used for main effects and interactions involving factors with more than two levels.
Table 2.
Mean Startle Magnitude Before and After Treatment at Baseline and During Cue and ITI
| Baseline
|
Neutral
|
Predictable
|
Unpredictable
|
|||||
|---|---|---|---|---|---|---|---|---|
| Hab Pretreatment | Prethreat Posttreatment | ITI | Cue | ITI | Cue | ITI | Cue | |
| T Scores | ||||||||
| Placebo | 51.9 (1.7) | 55.9 (2.6) | 46.4 (.7) | 45.8 (.8) | 48.3 (.8) | 53.7 (1.7) | 50.8 (.9) | 51.4 (1.4) |
| Hydrocortisone (20 mg) | 57.0 (2.2) | 59.2 (2.8) | 47.1 (.7) | 46.7 (.5) | 49.2 (.7) | 55.4 (.9) | 51.8 (.8) | 54.1 (1.1) |
| Hydrocortisone (60 mg) | 55.8 (1.7) | 57.7 (1.9) | 46.2 (.6) | 46.8 (.8) | 50.0 (.8) | 54.8 (1.0) | 52.5 (.9) | 54.3 (1.1) |
| Raw Scores | ||||||||
| Placebo | 38.9 (8.2) | 49.4 (10.9) | 26.2 (4.8) | 24.8 (4.8) | 30.8 (5.3) | 44.1 (7.2) | 36.8 (5.3) | 39.0 (6.4) |
| Hydrocortisone (20 mg) | 31.0 (7.1) | 54.3 (11.3) | 29.0 (6.3) | 27.9 (5.8) | 35.1 (6.7) | 49.7 (7.7) | 40.7 (6.5) | 47.1 (7.4) |
| Hydrocortisone (60 mg) | 54.3 (10.0) | 51.2 (8.6) | 26.9 (6.3) | 28.3 (6.1) | 36.8 (7.2) | 48.8 (8.7) | 43.5 (8.2) | 47.5 (8.3) |
Mean (SEM) startle magnitude (μV). ITI, intertrial interval; Hab, habituation.
Results
Startle Magnitude
Table 2 shows the mean startle magnitude of the first four startle stimuli during startle habituation; the mean startle magnitude of the four startle stimuli preceding the first threat block (prethreat startle); and startle magnitude during ITI and during the cues in the no-shock, predictable, and unpredictable conditions.
Baseline Startle
Data for one subject during startle habituation are not included in this analysis, due to technical difficulties. Baseline startle (Table 2) was not affected by hydrocortisone. A Drug (placebo, hydrocortisone/low, hydrocortisone/high) × Time (pretreatment, prethreat) ANOVA conducted on the baseline startle magnitude scores revealed a Time effect [F (1,20) = 4.4, p < .05], reflecting increased startle during prethreat (i.e., after treatment) compared with pretreatment in all three treatment conditions. This effect possibly reflected anticipatory anxiety before the threat experiment. More importantly, no other main effect or interaction was significant (all p > .1), suggesting that cortisol did not affect baseline startle.
Cued Fear-Potentiated Startle
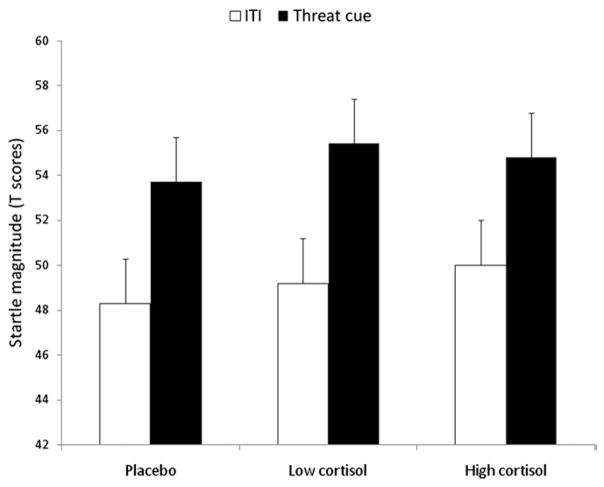
To examine fear-potentiated startle, startle magnitudes during ITI and during the cue in N and P across treatments were analyzed with a Stimulus Type (cue, ITI) × Condition (N, P) × Drug (3) ANOVA. As expected, there was a Stimulus Type × Condition interaction [F (1,21) = 99.1, p < .00009], reflecting larger startle during the cue relative to ITI in P compared with N. This interaction was not affected by hydrocortisone [non-significant Stimulus Type × Condition interaction × Drug interaction, F (2,42) = 2.0, p = ns, GG-ε = .98]. An analysis restricted to P (Figure 2) confirmed the lack of effect of hydrocortisone on cued fear-potentiated startle [Drug: F (2,42) = .6, p = ns, GG-ε = .95], including when the analysis was restricted to the high hydrocortisone dose [F (1,21) = .3, p = ns]. Similar results were obtained with the raw scores (Supplement 1).
Figure 2.
Startle magnitude to the threat cue and during intertrial interval (ITI) in the predictable shock condition. Fear-potentiated startle, the increased startle magnitude from ITI to the threat cue, was not affected by treatment.
Anxiety-Potentiated Startle
Anxiety-potentiated startle was examined with ITI startle magnitude with a Drug (3) × Condition (N, P, U) ANOVA. As expected (30), startle magnitude during ITI increased progressively from the N to the P to the U condition (Figure 3, Table 2), resulting in a condition linear trend [F (1,21) = 87.0, p < .0001]. This increase was differentially affected by hydrocortisone and placebo, as reflected by a significant Drug × Condition linear trend [F (1,21) = 4.2, p < .05]. To clarify this interaction, follow-up tests compared each active treatment with placebo. The high [F (2,42) =3.7, p <.03, GG-ε=.88] but not the low [F (2,42) =.8, p = ns, GG-ε = .75] dose of hydrocortisone increased startle potentiation. The high dose of hydrocortisone increased anxiety-potentiated startle in both P [F (1,21) = 6.1, p < .02] and U [F (1,21) = 4.2, p < .05]. Similar results were obtained with the raw scores (Supplement 1).
Figure 3.
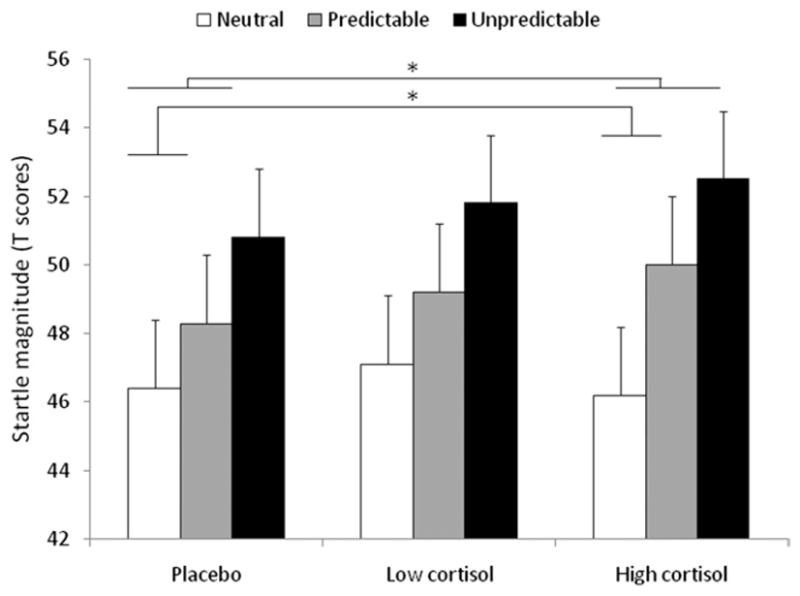
Startle magnitude during ITI in the N, P, and U conditions in each treatment. Anxiety-potentiated startle in P and U is the increased startle magnitude from the N to the P and from the N to the U condition, respectively. Anxiety-potentiated startle was increased by the high dose of hydrocortisone in the P and U conditions. *Significant (p < .05) effect. Abbreviations as in Figures 1 and 2.
Anxiety over Time
Fear and anxiety were operationally defined by their duration (i.e., short- vs. long-duration). However, what constitutes “short” and “long” is unclear. Hydrocortisone did not affect fear evoked 4–7 sec after the onset of threat cues in P. Was hydrocortisone also ineffective in affecting anxiety early on after the beginning of the P and U conditions? We did not test anxiety-potentiated startle 4–7 sec after the beginning of the P and the U conditions, but the design of the study was such that anxiety-potentiated startle was tested at three different times: early (15–52 sec), middle (53–96 sec), and late (97–140 sec) in P and U (see Methods and Materials). In a post hoc analysis, we investigated whether the high dose of hydrocortisone enhanced anxiety to the same degree throughout P and U or whether startle potentiation was insensitive to hydrocortisone effect at the earliest time. The placebo and high hydrocortisone data were reanalyzed, taking into account the time of startle delivery during each condition. To reduce the variability of startle magnitude and because there were no differences in the effect of hydro-cortisone on anxiety in P and U, we averaged these two conditions together. The data were then analyzed with a Drug (placebo, high hydrocortisone) × Condition (N, mean of P and U) × Time (early, middle, late) ANOVA. Figure 4 shows that the hydrocortisone-induced enhancement of startle magnitude in P/U compared with N did not differ across the three time points, suggesting that hydrocortisone increased potentiated startle as early as 15–50 sec after the onset of N and U. This was confirmed statistically; the Condition × Drug interaction was significant [(F (1,21) = 7.0, p < .02], but the Condition × Drug × Time interaction was not [(F (2,42) = .6, p > .5, GG-ε = .91].
Figure 4.
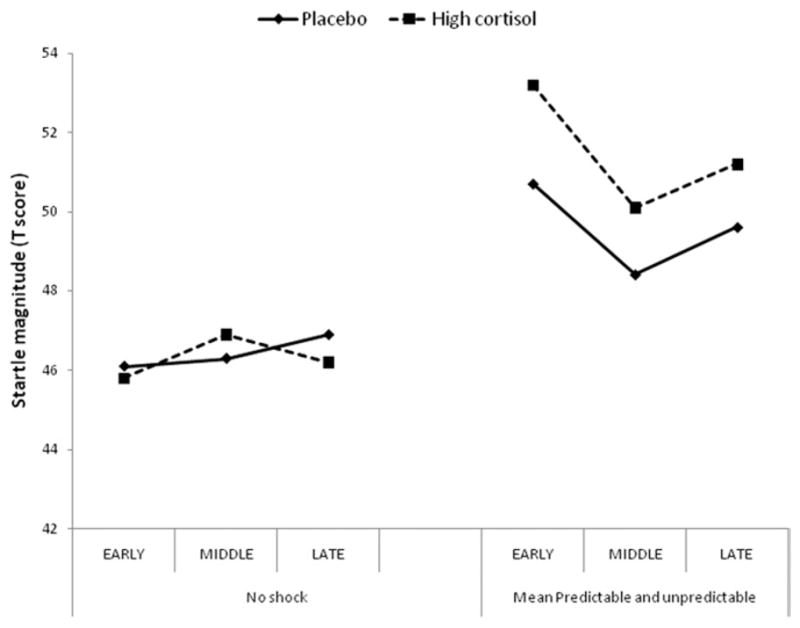
Time course of anxiety-potentiated startle in the early, middle, and late phase of each condition. Startle magnitude during the predictable and unpredictable conditions were averaged together to reduce variability.
Subjective Anxiety, State Anxiety, and Pain
The retrospective ratings of anxiety, the state anxiety scores, and the pain measures were not significantly affected by hydrocortisone (Supplement 1).
Salivary Cortisol
As expected, hydrocortisone increased cortisol levels dose-dependently (Table 3). The cortisol data were analyzed with a Drug (3) × Time (5) ANOVA. There were significant main effects of Drug [F (2,42) = 86.7, p < .0001, GG-ε = .34] and Time [F (4,84) = 46.6, p < .0001, GG-ε = .34] as well as a Drug × Time interaction [F (8,168) = 35.0, p < .0001, GG-ε = .34]. The interaction reflected increased cortisol levels after cortisol administration (high hydrocortisone > low hydrocortisone > placebo; all p > .0009).
Table 3.
Mean Cortisol Before and After Administration
| Predrug Baseline 1 | Predrug Baseline 2 | Postdrug After 1st Threat Block | Postdrug After 2nd Threat Block | Postdrug Posttest | |
|---|---|---|---|---|---|
| Placebo | .16 (.01) | .17 (.02) | .13 (.02) | .13 (.01) | .10 (.02) |
| Hydrocortisone (20 mg) | .17 (.02) | .17 (.05) | 1.79 (.29) | 1.76 (.31) | 1.79 (.34) |
| Hydrocortisone (60 mg) | .18 (.02) | .16 (.01) | 5.66 (.76) | 6.7 (.71) | 6.45 (.76) |
Mean (SEM) cortisol (ng/mL).
Discussion
Although evidence suggests that chronic as well as acute glucocorticoids are anxiogenic in animals (2–5,14,16), much remains to be learned about their emotional effects in humans. A key question is whether cortisol sensitizes anxiety or is involved in its termination. The present results indicate that cortisol increases anxiety. Specifically, we examined the effect of acute hydrocortisone treatment on two types of potentiated startle responses, fear-potentiated startle evoked by a short-duration threat cue and anxiety-potentiated startle associated with longer-duration contextual threat. Results showed that the high dose of hydrocortisone (60 mg) increased anxiety-potentiated startle without affecting fear-potentiated startle. The results showing that cortisol increases aversive states are consistent with the observation that increased endogenous cortisol levels are associated with negative affect and with animal (33) and human (34) data showing an anxiogenic effect of cortisol on potentiated startle.
That glucocorticoids are anxiogenic is also in line with findings that blockade of glucocorticoid synthesis reduces anxiety in humans. Indeed, the preclinical finding that inhibition of steroid synthesis reduces both corticosterone levels and anxiety in rats exposed to a predator (cat) (35) has prompted similar investigations in humans. The effect of the cortisol synthesis inhibitor metyrapone was evaluated in patients with panic disorders. In one study, metyrapone nonsignificantly reduced anxiety symptoms in the healthy comparison group (36). In another study, which used a panicogenic carbon dioxide challenge, metyrapone modestly reduced anxiety during the period that preceded the panicogenic challenge in the patients without affecting the panic symptoms to the challenge itself, suggesting a differential effect of cortisol on fear and generalized anxiety symptoms (37). These results are consistent with the present findings that hydrocortisone increased anxiety but not fear.
These findings point to an anxiogenic effect of hydrocortisone, but contradictory results have been reported. Hydrocortisone decreases negative mood (38,39), but null effect (40,41) as well as increased negative mood (7,42–44) have also been found. One possibility is that hydrocortisone lessens negative mood during mild stressors, but this effect might be subtle and dose-dependent (38,39). A relatively low dose of hydrocortisone (30 mg) affects fear conditioning, decreasing it in male subjects and increasing it in female subjects (45–47). A reanalysis of our data did not reveal any trend for a gender effect on anxiety responses. It is possible that hydrocortisone did not affect the expression of fear per se in these conditioning studies but influenced any of the multitudes of processes involved in conditioning, such as attention, learning, and memory. This latter interpretation is consistent with findings that cortisol decreases phobic symptoms and might improve symptoms of post-traumatic stress disorder (9,48)—not by alleviating the expression of fear per se but by preventing the retrieval of “vivid and excessive stimulus-associated fear memory” that led to the phobic/traumatic response (9,48). If cortisol indirectly reduces (or increases) fear and anxiety by interfering with emotional memory processes, such a reduction would not be expected in our study because fear and anxiety induction did not rely on memory. Indeed, the study was explicitly designed to examine the expression of fear and anxiety as opposed to aversive learning and memory; each safe and threat condition was clearly signaled to the subjects on a monitor. It is thus possible that cortisol affects multiple emotional processes; cortisol might both impair emotional memory retrieval and increase the expression of anxiety, the former effect being more likely at low doses, and the latter one being more likely at high doses.
What are the potential mechanisms for the cortisol-induced increase in anxiety-potentiated startle? The present study was based on the premise (see introductory text) that: 1) anxiety is mediated by activation of CRH receptors in the BNST (17), and 2) cortisol increases extrahypothalamic CRH (16). Cortisol and CRH might have worked together to enhance anxiety, cortisol potentiating the effect of CRH in the BNST. This interpretation is supported by findings that corticosterone administered in the BNST increases anxiety-like behaviors in the rat (16). Accordingly, the failure of cortisol to affect fear-potentiated startle to the threat cue is consistent with the finding that cued fear-potentiated startle in rodents is not affected by CRH (19).
An alternative possibility is that cortisol influenced brain areas involved in the processing of contextual cues, as opposed to explicit threat cues. Because the hippocampus is rich in glucocorticoid receptors and is essential for contextual processing, this structure might be involved in the present findings. In fact, prior studies have found a selective effect of corticosterone on cue and context conditioning in rodents, possibly due to increased cortisol-induced excitability of the hippocampus (49). A better comprehension of glucocorticoid effects will require an understanding of the effect of this steroid hormone on various constituents of aversive states.
Little is known about the effect of cortisol on startle in humans. Consistent with our results, past studies showed no significant modulation of baseline startle of 4 days of prednisone treatment (160 mg/day) (50) or acute treatment with cortisol (5 mg, 20 mg) compared with placebo (40). These two studies also showed no effect of cortisol on the modulation of startle by affective picture. These results suggest that, within the range of doses studied so far, cortisol does not affect baseline startle and potentiated response to mildly aversive stimuli but increases the potentiation of startle to more evocative and long-lasting threats.
The present results need to be interpreted in the context of its strengths and limitations. The main strength of this study is its reliance on a robust translational approach with a well-developed and well-proven procedure. One limitation is the relatively small sample size. However, this sample size is similar to or greater than that of our previous psychopharmacological studies (29,30,51). In addition, we used a within-subjects design, which improves statistical power. Another limitation was that the effect of hydrocortisone on potentiated startle was not found with the subjective anxiety data. Reports of dissociation between objective measures and subjective reports are frequent in drug studies (51–53). The most likely reason for the differential effect of hydrocortisone on physiological and subjective reports in the present study is that startle was used to probe anxiety online, whereas the subjective anxiety measures were retrospective. Subtle differences in responding might have been affected by the passage of time and by the complexity of the design. Finally, it is highly likely that startle potentiation and subjective reports reflect the influence of different structures, subjective report being more cortically mediated than startle.
This study found that acute hydrocortisone increased anxiety without affecting fear. These results raise concerns as to the use of cortisol to treat anxiety (9,54). Cortisol might reduce fear by interfering with retrieval of emotional memory (9), but it might also increase the expression of anxiety. There is growing evidence from animal studies that activation of CRH receptors in the BNST mediates sustained anxiety. Increased anxiety in the present study could therefore be due to a potentiation of CRH activity in the BNST by cortisol. This hypothesis, however, cannot be tested in humans. A significant advantage of our experimental model is its cross-species nature. Future studies in animals should examine the role of acute glucocorticoids in sustained anxiety states and, more particularly, whether any effect is dependent on CRH activity.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health.
Footnotes
Supplementary material cited in this article is available online.
The author(s) declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. Dr. Pine has received compensation for activities related to teaching, editing, and clinical care that pose no conflicts of interest.
References
- 1.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- 3.File SE, Vellucci SV, Wendlandt S. Corticosterone—An anxiogenic or an anxiolytic agent? J Pharm Pharmacol. 1979;31:300–305. doi: 10.1111/j.2042-7158.1979.tb13505.x. [DOI] [PubMed] [Google Scholar]
- 4.Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav. 2004;82:751–762. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behav Brain Res. 2004;149:209–215. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- 7.Warburton DM. Modern biochemical concepts of anxiety. Implications for psychopharmacological treatment. Int Pharmacopsych. 1974;9:189–205. doi: 10.1159/000468134. [DOI] [PubMed] [Google Scholar]
- 8.Putman P, Hermans EJ, Koppeschaar H, van Schijndel A, van Honk J. A single administration of cortisol acutely reduces preconscious attention for fear in anxious young men. Psychoneuroendocrinology. 2007;32:793–802. doi: 10.1016/j.psyneuen.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, et al. Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci U S A. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perksy H, Smith KD, Basu GK. Effect of corticosterone and hydrocortisone on some Indicators of Anxiety. J Clin Endocrinol Metab. 1971;33:467–473. doi: 10.1210/jcem-33-3-467. [DOI] [PubMed] [Google Scholar]
- 11.Makino S, Gold PW, Schulkin J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res. 1994;657:141–149. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- 12.Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: A hybridization histochemical study in the rat. J Comp Neurol. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- 13.Muller MB, Zimmermann S, Sillaber I, Hagemeyer T, Deussing JM, Timpl P, et al. Limbic corticotropin releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- 14.Schulkin J, Morgan MA, Rosen JB. A neuroendocrine mechanism for sustaining fear. Trends Neurosci. 2005;28:629–635. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Merali Z, Anisman H, James J, Kent P, Schulkin J. Effects of corticosterone on corticotrophin-releasing hormone and gastrin-releasing peptide release in response to an aversive stimulus in two regions of the forebrain (central nucleus of the amygdala and prefrontal cortex) Eur J Neurosci. 2008;28:165–172. doi: 10.1111/j.1460-9568.2008.06281.x. [DOI] [PubMed] [Google Scholar]
- 16.Shepard JD, Chambers CO, Busch C, Mount A, Schulkin J. Chronically elevated corticosterone in the dorsolateral bed nuclei of stria terminalis increases anxiety-like behavior. Behav Brain Res. 2009;203:146–149. doi: 10.1016/j.bbr.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillon C. Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacologie. 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker DL, Davis M. Light-enhanced startle: Further pharmacological and behavioral characterization. Psychopharmacology(Berl) Berl. 2002;159:304–310. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- 20.Watts AG, Sanchez-Watts G. Region-specific regulation of the neuropeptide mRNAs in rat limbic forebrain neurons by aldosterone and corticosterone. J Physiol. 1995;484:721–736. doi: 10.1113/jphysiol.1995.sp020698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otto T, Poon P. Dorsal hippocampal contributions to unimodal contextual conditioning. J Neurosci. 2006;26:6603–6609. doi: 10.1523/JNEUROSCI.1056-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grillon C, Baas JMP, Cornwell BR, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: Effect of predictability. Biol Psychiatry. 2006;60:752–759. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 23.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Vansteenwegen D, Iberico C, Vervliet B, Marescau V, Hermans D. Contextual fear induced by unpredictability in a human fear conditioning preparation is related to the chronic expectation of a threatening US. Biol Psychol. 2008;77:39–46. doi: 10.1016/j.biopsycho.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- 26.Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- 27.Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in post-traumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grillon C, Chavis C, Covington MS, Pine DS. Two-week treatment with citalopram reduces contextual anxiety but not cued fear. Neuropsychopharmacology. 2009;34:964–971. doi: 10.1038/npp.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 31.First MB, Spitzer RI, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-V (SCID) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 32.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, California: Consulting Psychologist Publishing Group; 1983. [Google Scholar]
- 33.Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: Behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–1104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- 34.Grillon C, Pine DS, Baas JMP, Ellis V, Charney DS. Cortisol and DHEA-S are associated with startle potentiation during aversive conditioning in humans. Psychopharmacologie. 2006;186:434–441. doi: 10.1007/s00213-005-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen HH, Benjamin JJ, Kaplan ZZ, Kotler MM. Administration of high-dose ketoconazole, an inhibitor of steroid synthesis, prevents posttraumatic anxiety in an animal model. Eur Neuropsychopharmacol. 2000;10:429–435. doi: 10.1016/s0924-977x(00)00105-x. [DOI] [PubMed] [Google Scholar]
- 36.Kellner M, Schick M, Yassouridis A, Struttmann T, Wiedemann K, Alm B. Metyrapone tests in patients with panic disorder. Biol Psychiatry. 2004;56:898–900. doi: 10.1016/j.biopsych.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Belgorodsky A, Knyazhansky L, Loewenthal U, Arbelle J, Cohen H, Benjamin J. Effects of the cortisol synthesis inhibitor metyrapone on the response to carbon dioxide challenge in panic disorder. Depress Anxiety. 2005;21:143–148. doi: 10.1002/da.20062. [DOI] [PubMed] [Google Scholar]
- 38.Het S, Wolf OT. Mood changes in response to psychosocial stress in healthy young women: Effects of pretreatment with cortisol. Behav Neurosci. 2007;121:11–20. doi: 10.1037/0735-7044.121.1.11. [DOI] [PubMed] [Google Scholar]
- 39.Reuter M. Impact of cortisol on emotions under stress and non-stress conditions: A pharmacopsychological approach. Neuropsychobiology. 2002;46:41–48. doi: 10.1159/000063575. [DOI] [PubMed] [Google Scholar]
- 40.Buchanan TW, Brechtel A, Sollers JJ, Lovallo WR. Exogenous cortisol exerts effects on the startle reflex independent of emotional modulation. Pharmacol Biochem Behav. 2001;68:203–210. doi: 10.1016/s0091-3057(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 41.Monk C, Nelson C. The effects of hydrocortisone on cognitive and neural function: A behavioral and event-related potential investigation. Neuropsychopharmacology. 2002;26:505–519. doi: 10.1016/S0893-133X(01)00384-0. [DOI] [PubMed] [Google Scholar]
- 42.Weiner S, Dorman D, Levitt E, Stach T, Persky H, Norton J. Effect on anxiety of increasing plasma hydrocortisone level. Psychosom Med. 1963;25:69. [Google Scholar]
- 43.Levitt EE, Persky H, Brady JP, Fitzgerald JA. The effect of hydorcotisone infusion on hypnotically induced anxiety. Psychosom Med. 1963;25:158–161. doi: 10.1097/00006842-196303000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Persky H, Smith KD, Basu GK. Effect of corticosterone and hydrocortisone on some Indicators of Anxiety. J Clin Endocrinol Metab. 1971;33:467–473. doi: 10.1210/jcem-33-3-467. [DOI] [PubMed] [Google Scholar]
- 45.Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, et al. Influence of the stress hormone cortisol on fear conditioning in humans: Evidence for sex differences in the response of the prefrontal cortex. Neuroimage. 2006;32:1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 46.Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology. 2010;35:33–46. doi: 10.1016/j.psyneuen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Tabbert K, Merz CJ, Klucken T, Schweckendiek J, Vaitl D, Wolf OT, Stark R. Cortisol enhances neural differentiation during fear acquisition and extinction in contingency aware young women. Neurobiol Learn Mem. 2010;94:392–401. doi: 10.1016/j.nlm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 48.de Quervain DJ, Margraf J. Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: A novel therapeutic approach. Eur J Pharmacol. 2008;583:365–371. doi: 10.1016/j.ejphar.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 49.Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111:503–511. [PubMed] [Google Scholar]
- 50.Schmidt LA, Fox NA, Goldberg MC, Smidth CC, Schulkin J. Effects of acute prednisone administration on memory, attention, and emotion in healthy human adults. Psychoneuroendocrinololy. 1999;24:461–483. doi: 10.1016/s0306-4530(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 51.Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: A fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- 52.Harmer CJ, Rogers RD, Tunbridge E, Cowen PJ, Goodwin GM. Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacologie. 2003;167:411–417. doi: 10.1007/s00213-003-1401-6. [DOI] [PubMed] [Google Scholar]
- 53.Kemp AH, Gray MA, Silberstein RB, Armstrong SM, Nathan PJ. Augmentation of serotonin enhances pleasant and suppresses unpleasant cortical electrophysiological responses to visual emotional stimuli in humans. Neuroimage. 2004;22:1084–1096. doi: 10.1016/j.neuroimage.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 54.Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.