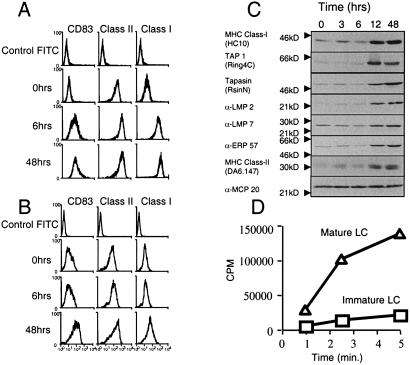
Figure 2.
LPS induced changes in the MHC class I antigen presentation pathway in DCs. Immature CD34-derived LCs (A) and monocyte-derived DCs (B) were activated by addition of 0.5 μg of LPS. At time 0, 6, and 48 h post-LPS, cells were harvested and fixed, and the expression of DC activation marker CD83, MHC class II, and MHC class I was monitored by flow cytometry. (C) LPS mediated a coordinate induction of MHC class I components. CD34-derived LCs were induced with LPS for 0–48 h, after which the cells were extracted in 1% Triton X-100, and membranes from separated lysates were probed with antibodies specific for the indicated proteins. MCP20 is a constitutive, noninducible component of the human proteasome and serves as a loading control for each time point. (D) LPS increases TAP-mediated peptide transport in CD34-derived LCs. Streptolysin O-permeabilized immature (□) and LPS-matured (▵) CD34-derived LCs were incubated with the iodinated RRYQNSTEL peptide at 37°C for the indicated time periods. Translocation was assessed by binding of the hot, glycosylated reporter peptide to Con A-Sepharose beads.