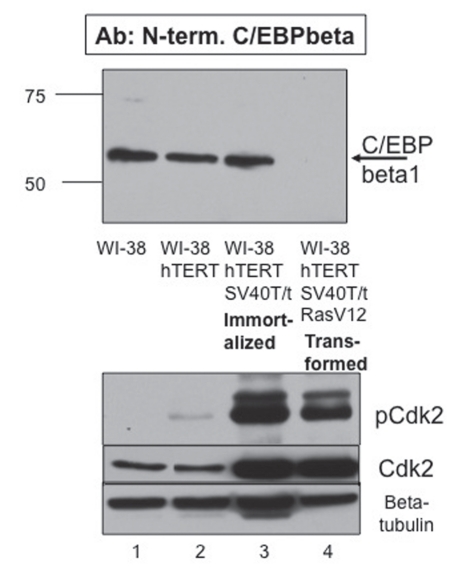
Figure 2.
Phosphorylation of C/EBPβ1 by Cdk2 is necessary but not sufficient for proteasomal degradation of C/EBPβ1. Top immunoblot—immunoblot analysis of WI-38, WI-38-hTERT, immortalized WI-38-hTERT-SV40T/t and transformed WI-38-hTERT-SV40T/t-Ras(V12) cells with the N-terminal C/EBPβ1-specific antibody described in Eaton et al. WI-38 cells were engineered via infection and subsequent selection with pBABE-hTERT-hygromycin (Addgene), pBABE-SV40T/t-neomycin (Addgene), and/or pBABE-Ras(V12)-puromycin. Cell lysates were prepared and analyzed via 10% SDS-PAGE. Bars indicate the mobility of standard molecular weight markers, in kilo-Daltons (kDa). Bottom immunoblots—the top part is the same samples with an anti-phosphoT160 Cdk2 antibody (Cell Signaling), the middle part is with an anti-Cdk2 antibody (Santa Cruz) and the bottom part is for β tubulin (Sigma) as a loading control.